 Found 9 hits Enzyme Inhibition Constant Data
Found 9 hits Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase FKBP1A
(106/107 > 99%)†
(Homo sapiens (Human)) | BDBM50030448
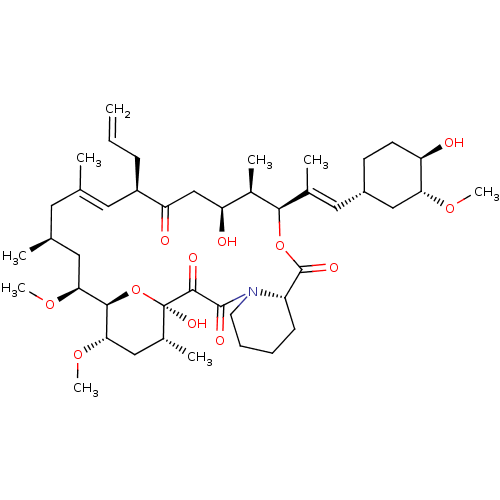
(8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...)Show SMILES CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]1C)OC |r,t:45| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(106/107 > 99%)†
(Homo sapiens (Human)) | BDBM50030448

(8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...)Show SMILES CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]1C)OC |r,t:45| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity against human FK506 binding protein 12 |
J Med Chem 42: 4456-61 (1999)
BindingDB Entry DOI: 10.7270/Q24J0DBT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(106/107 > 99%)†
(Homo sapiens (Human)) | BDBM50030448

(8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...)Show SMILES CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]1C)OC |r,t:45| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Amplyx Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human FKBP12 measured after 30 mins by fluorescein labelled SLF tracer based fluorescence polarization assay |
Bioorg Med Chem Lett 27: 2465-2471 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.004
BindingDB Entry DOI: 10.7270/Q20G3N9G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein phosphatase 3 catalytic subunit alpha
(374/375 > 99%)†
(Homo sapiens (Human)) | BDBM50079777
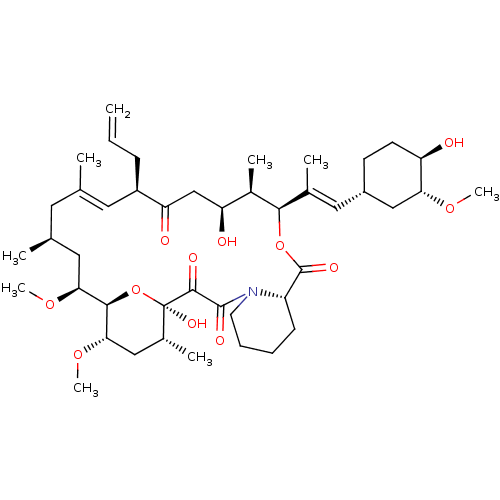
((E)-(1R,9S,12S,13R,14R,21S,23S,24R,25S,27R)-17-All...)Show SMILES [H][C@]12O[C@](O)([C@H](C)C[C@@H]1OC)C(=O)C(=O)N1CCCC[C@@]1([H])C(=O)O[C@@H]([C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)\C=C(C)\C[C@H](C)C[C@@H]2OC)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:39| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30?,31?,32-,33+,34+,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(106/107 > 99%)†
(Homo sapiens (Human)) | BDBM50030448

(8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...)Show SMILES CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]1C)OC |r,t:45| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinant FK506 binding protein 12 to determine the FKBP binding property of the compound |
J Med Chem 24: 496-9 (1981)
BindingDB Entry DOI: 10.7270/Q2K64JTV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(106/107 > 99%)†
(Homo sapiens (Human)) | BDBM50030448

(8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...)Show SMILES CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]1C)OC |r,t:45| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
| n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to FK506 binding protein 12 was determined |
Bioorg Med Chem Lett 5: 2341-2346 (1995)
Article DOI: 10.1016/0960-894X(95)00404-H
BindingDB Entry DOI: 10.7270/Q2JQ11HB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(106/107 > 99%)†
(Homo sapiens (Human)) | BDBM50030448

(8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...)Show SMILES CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]1C)OC |r,t:45| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
| n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for its affinity to the binding domain of immunophilin FK506 binding protein 12 |
Bioorg Med Chem Lett 3: 1947-1950 (1993)
Article DOI: 10.1016/S0960-894X(01)80992-6
BindingDB Entry DOI: 10.7270/Q2V40V3F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(106/107 > 99%)†
(Homo sapiens (Human)) | BDBM50079777

((E)-(1R,9S,12S,13R,14R,21S,23S,24R,25S,27R)-17-All...)Show SMILES [H][C@]12O[C@](O)([C@H](C)C[C@@H]1OC)C(=O)C(=O)N1CCCC[C@@]1([H])C(=O)O[C@@H]([C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)\C=C(C)\C[C@H](C)C[C@@H]2OC)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:39| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30?,31?,32-,33+,34+,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against FK506 binding protein 12 using [3H]-dihydro FK-506 |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein phosphatase 3 catalytic subunit alpha
(374/375 > 99%)†
(Homo sapiens (Human)) | BDBM50030448

(8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...)Show SMILES CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]1C)OC |r,t:45| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to protein phosphatase, calcineurin (CN) was determined |
Bioorg Med Chem Lett 5: 2341-2346 (1995)
Article DOI: 10.1016/0960-894X(95)00404-H
BindingDB Entry DOI: 10.7270/Q2JQ11HB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data