

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (503/503 = 100%)† (Homo sapiens (Human)) | BDBM8592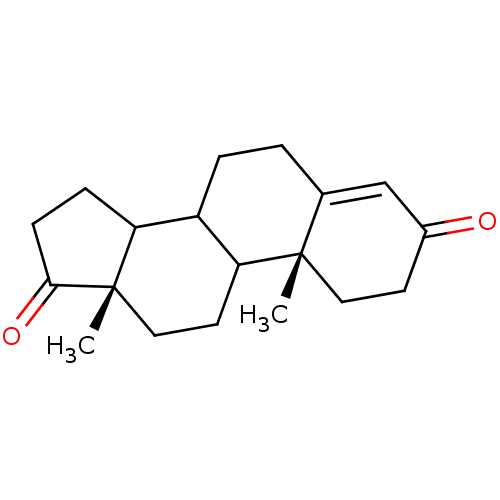 ((2R,15S)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 20 | -45.7 | 300 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (503/503 = 100%)† (Homo sapiens (Human)) | BDBM50025428 (10,13-Dimethyl-1,6,7,8,9,10,11,12,13,14,15,16-dode...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for aromatase cytochrome P45019A1 by analysis of Dixon plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (503/503 = 100%)† (Homo sapiens (Human)) | BDBM50025428 (10,13-Dimethyl-1,6,7,8,9,10,11,12,13,14,15,16-dode...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description In vitro competetitive inhibitory activity was measured on Cytochrome P450 19A1 of human placental microsomes | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||