

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50074715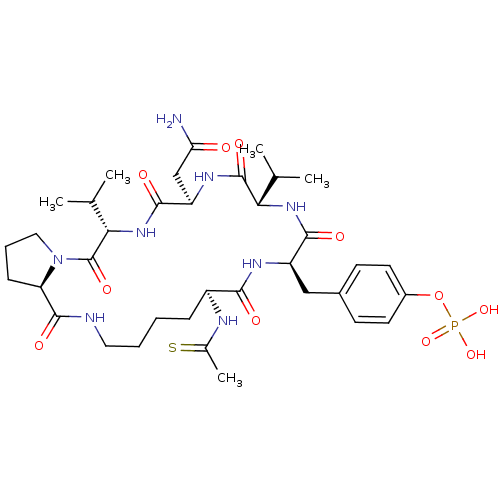 (CHEMBL433805 | Phosphoric acid mono-[4-((5S,8R,11S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Growth factor receptor bound protein 2 to biotinylated KPFY*VNVEF Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50074710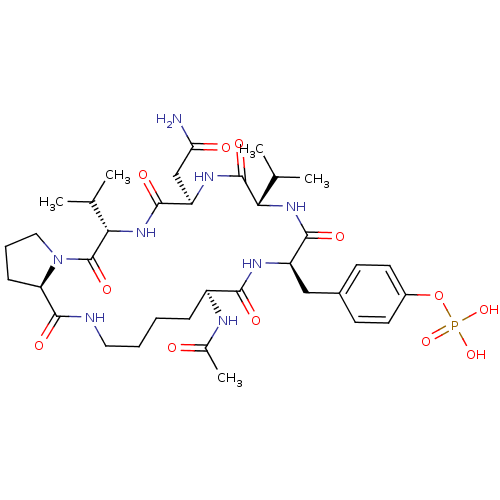 (CHEMBL435910 | Phosphoric acid mono-[4-((5S,8R,11S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Growth factor receptor bound protein 2 to biotinylated KPFY*VNVEF Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50074711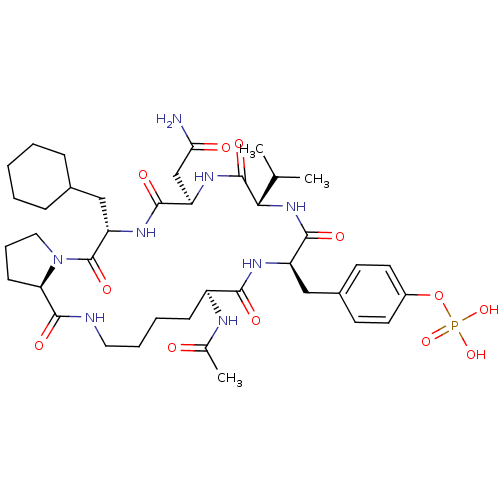 (CHEMBL355759 | Phosphoric acid mono-[4-((5S,8R,11S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Growth factor receptor bound protein 2 to biotinylated KPFY*VNVEF Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50074706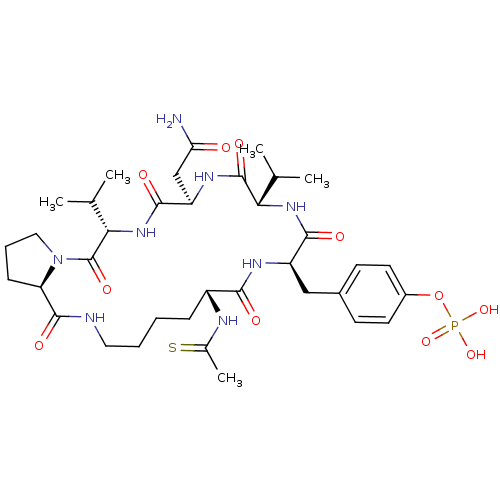 (CHEMBL366543 | Phosphoric acid mono-[4-((5S,8R,11S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Growth factor receptor bound protein 2 to biotinylated KPFY*VNVEF Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50074716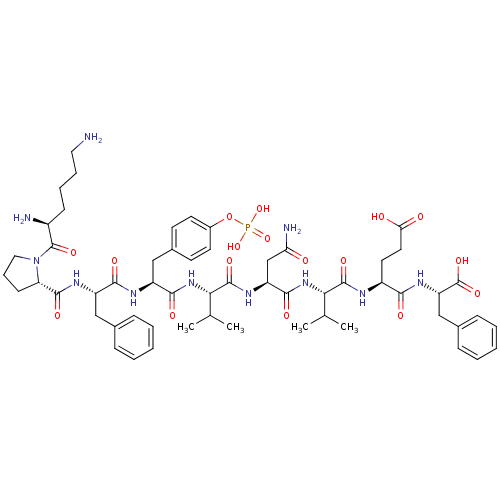 (CHEMBL438943 | Lys-Pro-Phe-pTyr-Val-Asn-Val-Glu-Ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Growth factor receptor bound protein 2 to biotinylated KPFY*VNVEF Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50074705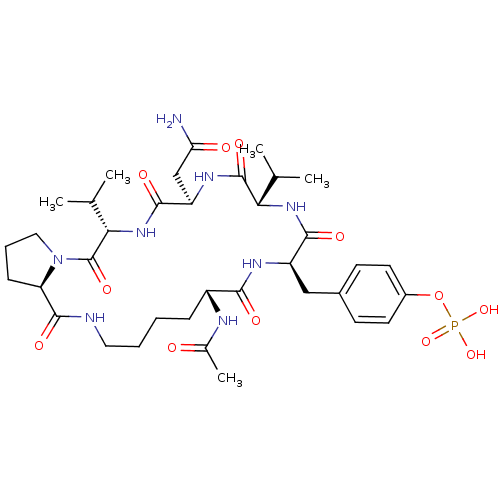 (CHEMBL359934 | Phosphoric acid mono-[4-((5S,8R,11S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Growth factor receptor bound protein 2 to biotinylated KPFY*VNVEF Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50074703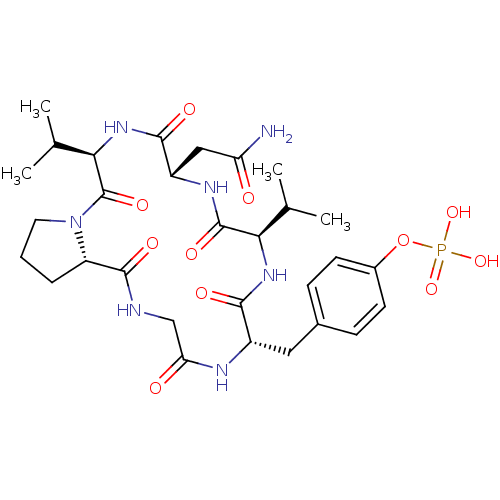 (CHEMBL172299 | Phosphoric acid mono-[4-((5R,8S,11R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Growth factor receptor bound protein 2 to biotinylated KPFY*VNVEF Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50074712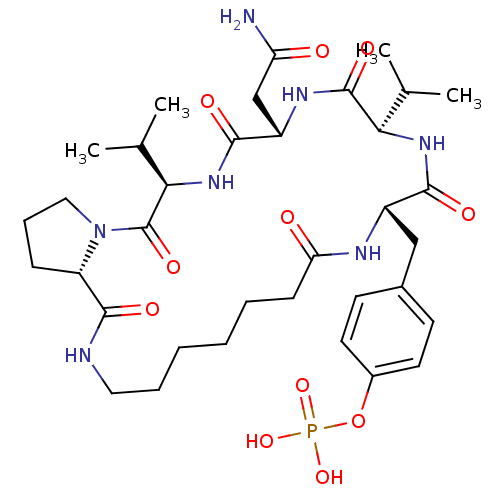 (CHEMBL177372 | Phosphoric acid mono-[4-((5R,8S,11R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Growth factor receptor bound protein 2 to biotinylated KPFY*VNVEF Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50074709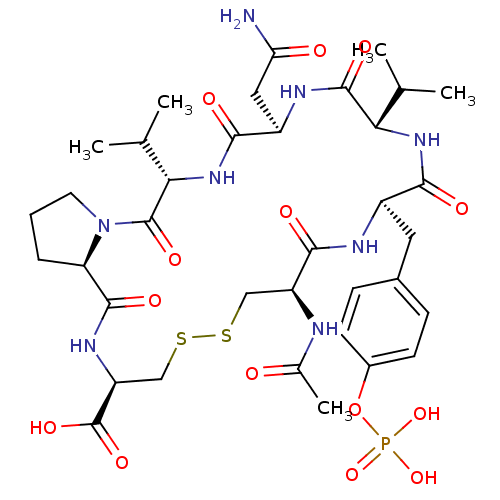 ((5S,8R,11S,14R,17R,22R,24aR)-17-Acetylamino-8-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Growth factor receptor bound protein 2 to biotinylated KPFY*VNVEF Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50074704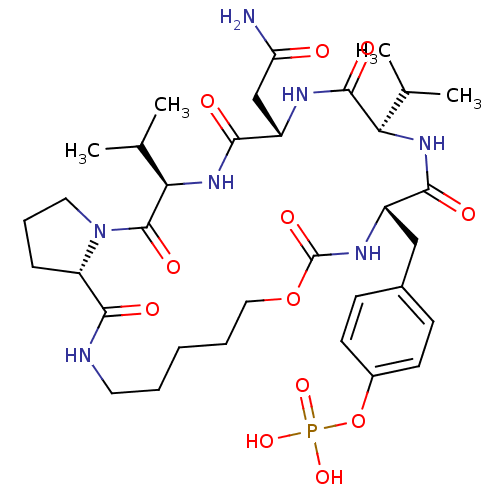 (CHEMBL435738 | Phosphoric acid mono-[4-((5R,8S,11R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Growth factor receptor bound protein 2 to biotinylated KPFY*VNVEF Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50074708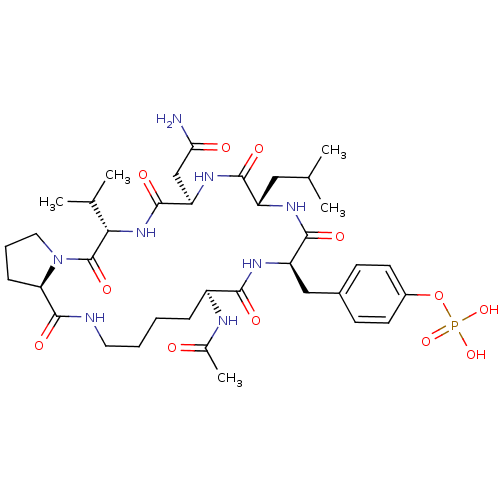 (CHEMBL177616 | Phosphoric acid mono-[4-((5S,8R,11S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Growth factor receptor bound protein 2 to biotinylated KPFY*VNVEF Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50074717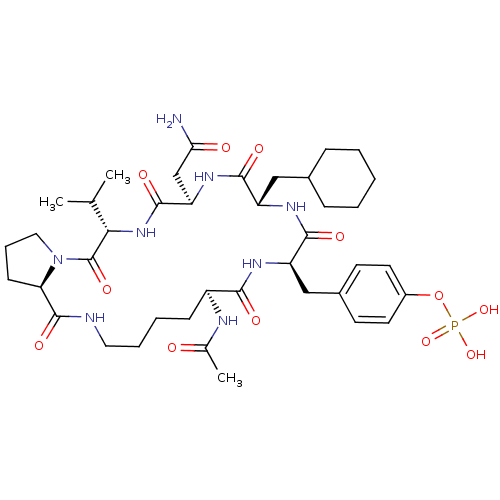 (CHEMBL451250 | Phosphoric acid mono-[4-((5S,8R,11S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Growth factor receptor bound protein 2 to biotinylated KPFY*VNVEF Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50074713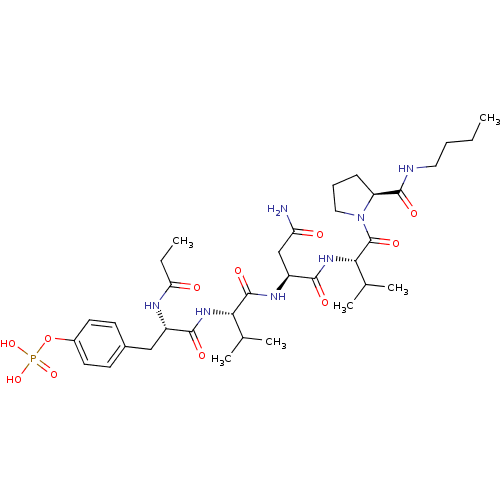 (CHEMBL367276 | Phosphoric acid mono-{4-[(S)-2-((S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Growth factor receptor bound protein 2 to biotinylated KPFY*VNVEF Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50074714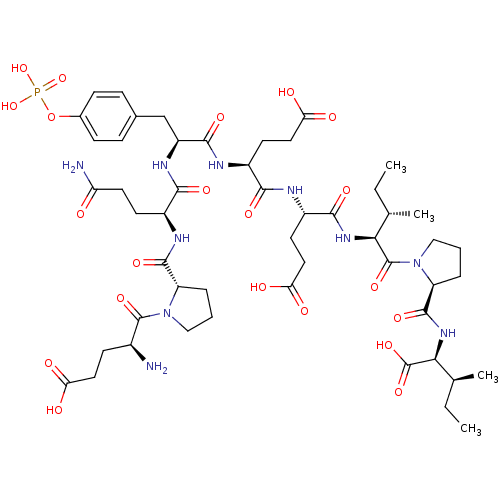 (CHEMBL263347 | Glu-Pro-Gln-pTyr-Glu-Glu-Ile-Pro-Il...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Growth factor receptor bound protein 2 to biotinylated KPFY*VNVEF Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50074707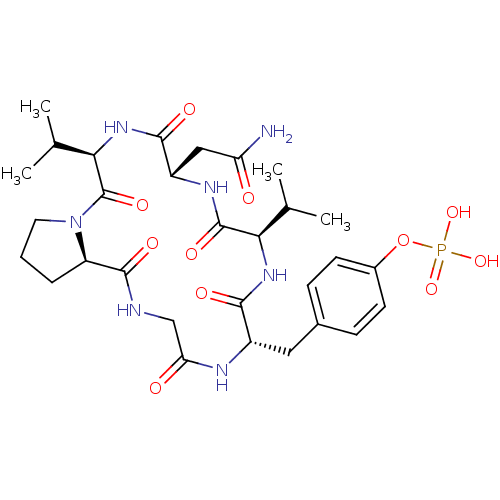 (CHEMBL174819 | Phosphoric acid mono-[4-((5R,8S,11R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Growth factor receptor bound protein 2 to biotinylated KPFY*VNVEF Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50074709 ((5S,8R,11S,14R,17R,22R,24aR)-17-Acetylamino-8-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Src protein tryrosine kinase SH2 domain to biotinylated EPQY*EEIPI Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50074715 (CHEMBL433805 | Phosphoric acid mono-[4-((5S,8R,11S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Src protein tryrosine kinase SH2 domain to biotinylated EPQY*EEIPI Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50074705 (CHEMBL359934 | Phosphoric acid mono-[4-((5S,8R,11S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Src protein tryrosine kinase SH2 domain to biotinylated EPQY*EEIPI Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50074706 (CHEMBL366543 | Phosphoric acid mono-[4-((5S,8R,11S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Src protein tryrosine kinase SH2 domain to biotinylated EPQY*EEIPI Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50074707 (CHEMBL174819 | Phosphoric acid mono-[4-((5R,8S,11R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Src protein tryrosine kinase SH2 domain to biotinylated EPQY*EEIPI Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50074704 (CHEMBL435738 | Phosphoric acid mono-[4-((5R,8S,11R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Src protein tryrosine kinase SH2 domain to biotinylated EPQY*EEIPI Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50074712 (CHEMBL177372 | Phosphoric acid mono-[4-((5R,8S,11R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Src protein tryrosine kinase SH2 domain to biotinylated EPQY*EEIPI Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50074703 (CHEMBL172299 | Phosphoric acid mono-[4-((5R,8S,11R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Src protein tryrosine kinase SH2 domain to biotinylated EPQY*EEIPI Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50074713 (CHEMBL367276 | Phosphoric acid mono-{4-[(S)-2-((S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Src protein tryrosine kinase SH2 domain to biotinylated EPQY*EEIPI Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50074711 (CHEMBL355759 | Phosphoric acid mono-[4-((5S,8R,11S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Src protein tryrosine kinase SH2 domain to biotinylated EPQY*EEIPI Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50074710 (CHEMBL435910 | Phosphoric acid mono-[4-((5S,8R,11S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Src protein tryrosine kinase SH2 domain to biotinylated EPQY*EEIPI Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50074717 (CHEMBL451250 | Phosphoric acid mono-[4-((5S,8R,11S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Src protein tryrosine kinase SH2 domain to biotinylated EPQY*EEIPI Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50074708 (CHEMBL177616 | Phosphoric acid mono-[4-((5S,8R,11S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Forschungsinstitut Curated by ChEMBL | Assay Description Inhibitory activity against binding of Src protein tryrosine kinase SH2 domain to biotinylated EPQY*EEIPI Peptide by ELISA. | J Med Chem 42: 971-80 (1999) Article DOI: 10.1021/jm9811007 BindingDB Entry DOI: 10.7270/Q28S4P35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||