

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50075121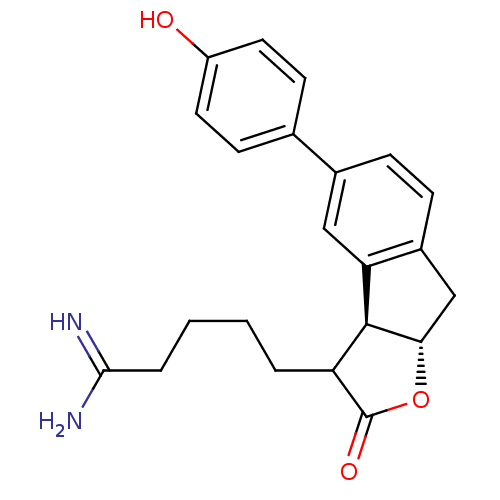 (5-[(3aS,8aS)-5-(4-Hydroxy-phenyl)-2-oxo-3,3a,8,8a-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin was determined | Bioorg Med Chem Lett 9: 431-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075122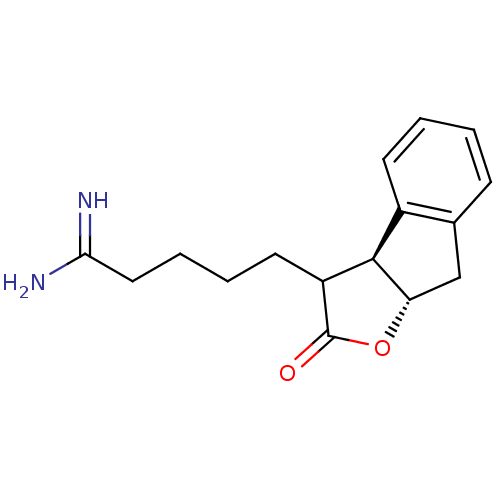 (5-((3aS,8aS)-2-Oxo-3,3a,8,8a-tetrahydro-2H-indeno[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin was determined | Bioorg Med Chem Lett 9: 431-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072293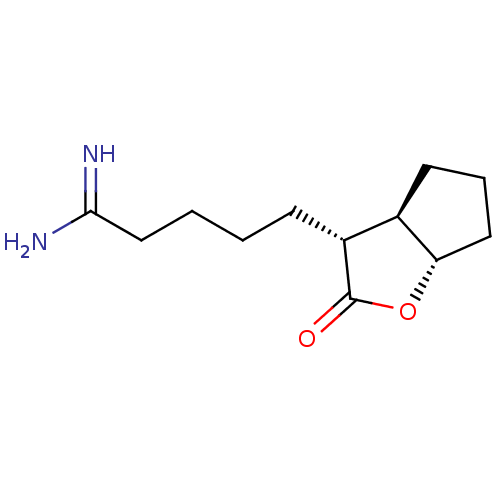 (5-((3R,3aR,6aS)-2-Oxo-hexahydro-cyclopenta[b]furan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin was determined | Bioorg Med Chem Lett 9: 431-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075123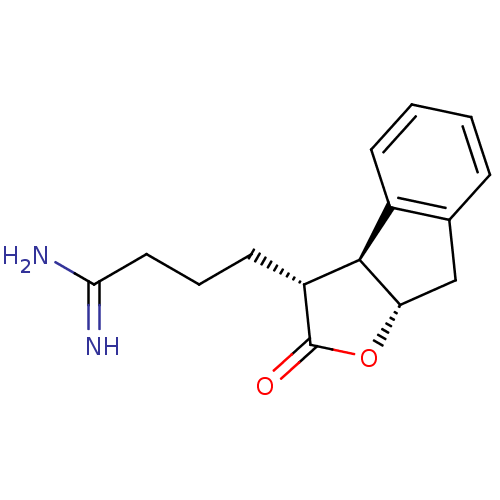 (4-((3R,3aS,8aS)-2-Oxo-3,3a,8,8a-tetrahydro-2H-inde...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin was determined | Bioorg Med Chem Lett 9: 431-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075120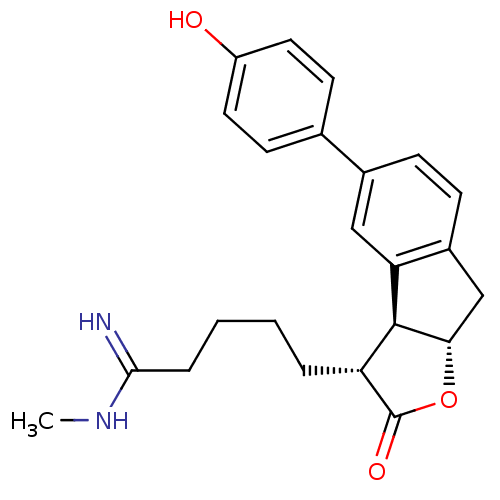 (5-[(3R,3aS,8aS)-5-(4-Hydroxy-phenyl)-2-oxo-3,3a,8,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin was determined | Bioorg Med Chem Lett 9: 431-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075119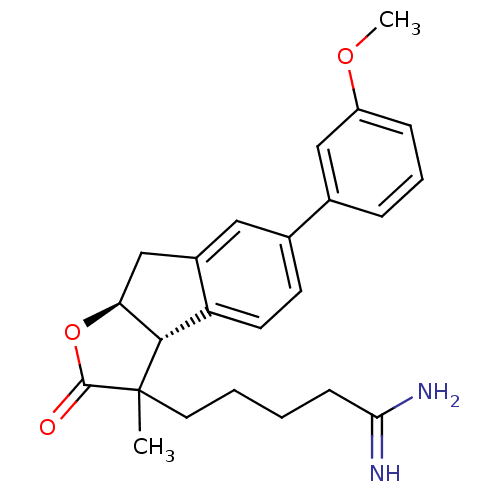 (5-[(3aR,8aS)-6-(3-Methoxy-phenyl)-3-methyl-2-oxo-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin was determined | Bioorg Med Chem Lett 9: 431-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||