

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein phosphatase 3 catalytic subunit alpha (Homo sapiens (Human)) | BDBM50079770 (C32-O-Phenalkyl ether derivative of Ascomycin | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for inhibition of serine/threonine protein phosphatase calcineurin (CAN) | Bioorg Med Chem Lett 9: 2085-8 (1999) BindingDB Entry DOI: 10.7270/Q22806SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 3 catalytic subunit alpha (Homo sapiens (Human)) | BDBM50079767 (C32-O-Phenalkyl ether derivative of Ascomycin | C3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for inhibition of serine/threonine protein phosphatase calcineurin (CAN) | Bioorg Med Chem Lett 9: 2085-8 (1999) BindingDB Entry DOI: 10.7270/Q22806SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50079766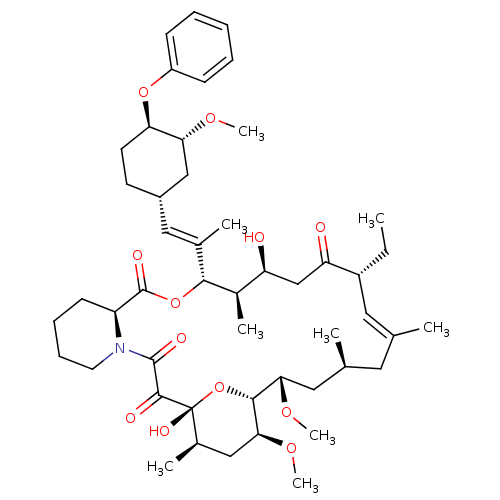 (17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-12-[2-(3-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration determined was towards FK506 binding protein 12 by competitive binding assay using [3H]-dihydro FK-506 as radioligand | Bioorg Med Chem Lett 9: 2085-8 (1999) BindingDB Entry DOI: 10.7270/Q22806SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50079765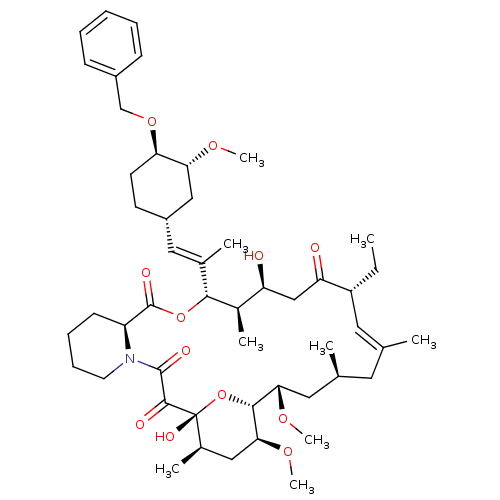 (C32-O-Phenalkyl ether derivative of Ascomycin | C3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration determined was towards FK506 binding protein 12 by competitive binding assay using [3H]-dihydro FK-506 as radioligand | Bioorg Med Chem Lett 9: 2085-8 (1999) BindingDB Entry DOI: 10.7270/Q22806SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50079770 (C32-O-Phenalkyl ether derivative of Ascomycin | CH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration determined was towards FK506 binding protein 12 by competitive binding assay using [3H]-dihydro FK-506 as radioligand | Bioorg Med Chem Lett 9: 2085-8 (1999) BindingDB Entry DOI: 10.7270/Q22806SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50079767 (C32-O-Phenalkyl ether derivative of Ascomycin | C3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration determined was towards FK506 binding protein 12 by competitive binding assay using [3H]-dihydro FK-506 as radioligand | Bioorg Med Chem Lett 9: 2085-8 (1999) BindingDB Entry DOI: 10.7270/Q22806SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50068939 ((E)-(9S,12S,13R,14S,17R,21S,23S,24R,25S,27S)-17-Et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration determined was towards FK506 binding protein 12 by competitive binding assay using [3H]-dihydro FK-506 as radioligand | Bioorg Med Chem Lett 9: 2085-8 (1999) BindingDB Entry DOI: 10.7270/Q22806SS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||