

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50083731 ((6R,9R)-10-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louisville Curated by ChEMBL | Assay Description Compound was tested for binding affinity against mu opioid receptor binding assay | Bioorg Med Chem Lett 9: 3441-6 (2000) BindingDB Entry DOI: 10.7270/Q2P55MQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50083732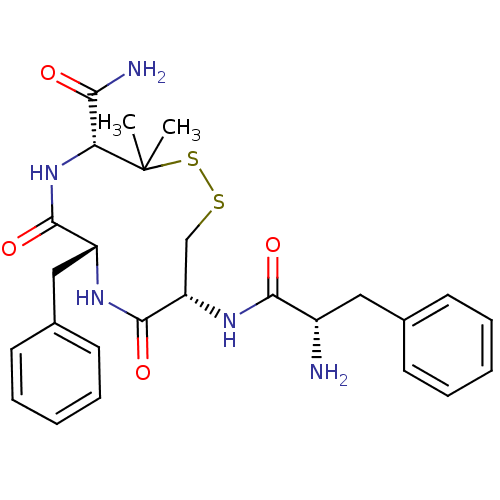 ((6R,9R)-10-((S)-2-Amino-3-phenyl-propionylamino)-7...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louisville Curated by ChEMBL | Assay Description Compound was tested for binding affinity against mu opioid receptor binding assay | Bioorg Med Chem Lett 9: 3441-6 (2000) BindingDB Entry DOI: 10.7270/Q2P55MQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50083734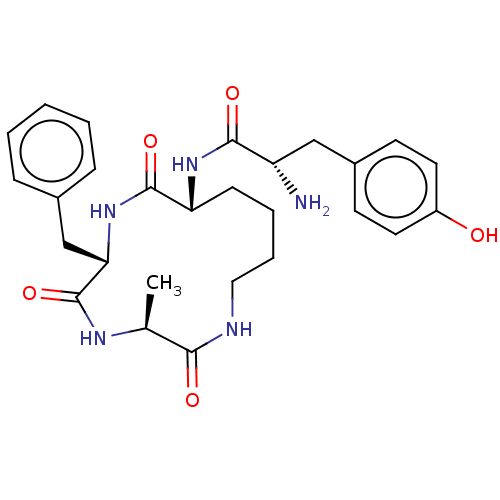 (2-Amino-N-[(R)-6-(4-hydroxy-benzyl)-3-methyl-2,5,8...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louisville Curated by ChEMBL | Assay Description In vitro mu opioid activity was determined by its ability to inhibit the electrically induced contractions of smooth muscle preparations in guinea pi... | Bioorg Med Chem Lett 9: 3441-6 (2000) BindingDB Entry DOI: 10.7270/Q2P55MQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50083734 (2-Amino-N-[(R)-6-(4-hydroxy-benzyl)-3-methyl-2,5,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louisville Curated by ChEMBL | Assay Description In vitro delta opioid activity was determined by its ability to inhibit the electrically induced contractions of smooth muscle preparations in mouse ... | Bioorg Med Chem Lett 9: 3441-6 (2000) BindingDB Entry DOI: 10.7270/Q2P55MQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50083732 ((6R,9R)-10-((S)-2-Amino-3-phenyl-propionylamino)-7...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louisville Curated by ChEMBL | Assay Description Compound was tested for its ability to activate mu opioid receptor in GPI bioassay | Bioorg Med Chem Lett 9: 3441-6 (2000) BindingDB Entry DOI: 10.7270/Q2P55MQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50083736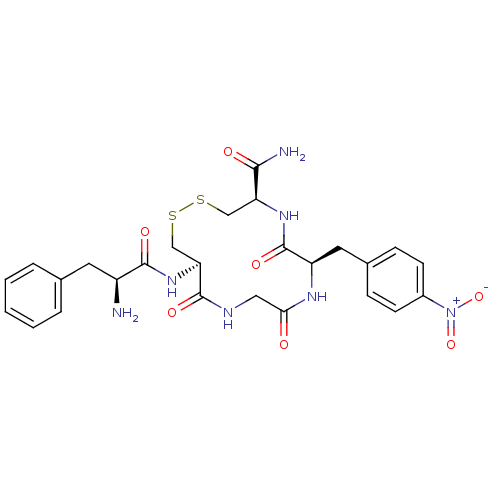 (13-((S)-2-Amino-3-phenyl-propionylamino)-7-(4-nitr...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louisville Curated by ChEMBL | Assay Description Compound was tested for moderate potency against mu opioid receptor in guinea pig ileum | Bioorg Med Chem Lett 9: 3441-6 (2000) BindingDB Entry DOI: 10.7270/Q2P55MQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50083730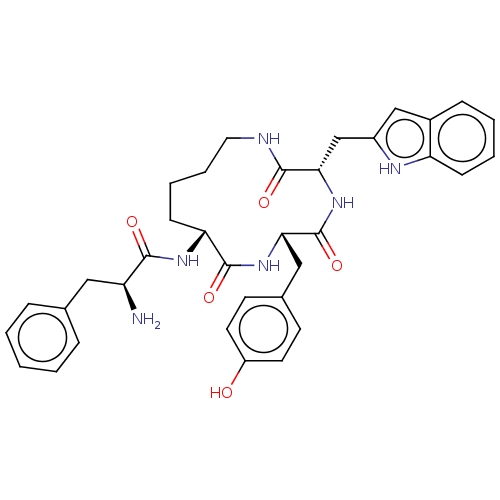 ((R)-2-Amino-N-[(S)-6-(4-hydroxy-benzyl)-3-(1H-indo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17.7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louisville Curated by ChEMBL | Assay Description In vitro delta opioid activity was determined by its ability to inhibit the electrically induced contractions of smooth muscle preparations in mouse ... | Bioorg Med Chem Lett 9: 3441-6 (2000) BindingDB Entry DOI: 10.7270/Q2P55MQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50083730 ((R)-2-Amino-N-[(S)-6-(4-hydroxy-benzyl)-3-(1H-indo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louisville Curated by ChEMBL | Assay Description In vitro mu opioid activity was determined by its ability to inhibit the electrically induced contractions of smooth muscle preparations in guinea pi... | Bioorg Med Chem Lett 9: 3441-6 (2000) BindingDB Entry DOI: 10.7270/Q2P55MQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50083729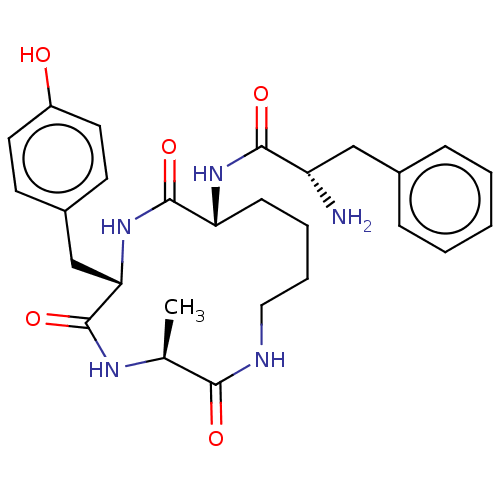 (2-Amino-N-((R)-6-benzyl-3-methyl-2,5,8-trioxo-1,4,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louisville Curated by ChEMBL | Assay Description In vitro mu opioid activity was determined by its ability to inhibit the electrically induced contractions of smooth muscle preparations in guinea pi... | Bioorg Med Chem Lett 9: 3441-6 (2000) BindingDB Entry DOI: 10.7270/Q2P55MQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50083729 (2-Amino-N-((R)-6-benzyl-3-methyl-2,5,8-trioxo-1,4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30.4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louisville Curated by ChEMBL | Assay Description In vitro delta opioid activity was determined by its ability to inhibit the electrically induced contractions of smooth muscle preparations in mouse ... | Bioorg Med Chem Lett 9: 3441-6 (2000) BindingDB Entry DOI: 10.7270/Q2P55MQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50083737 (13-((S)-2-Amino-3-phenyl-propionylamino)-7-benzyl-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louisville Curated by ChEMBL | Assay Description Compound was tested for moderate potency against mu opioid receptor in guinea pig ileum | Bioorg Med Chem Lett 9: 3441-6 (2000) BindingDB Entry DOI: 10.7270/Q2P55MQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50083733 (2-Amino-N-[(S)-6-(4-hydroxy-benzyl)-3-(1H-indol-2-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 627 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louisville Curated by ChEMBL | Assay Description In vitro mu opioid activity was determined by its ability to inhibit the electrically induced contractions of smooth muscle preparations in guinea pi... | Bioorg Med Chem Lett 9: 3441-6 (2000) BindingDB Entry DOI: 10.7270/Q2P55MQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50083735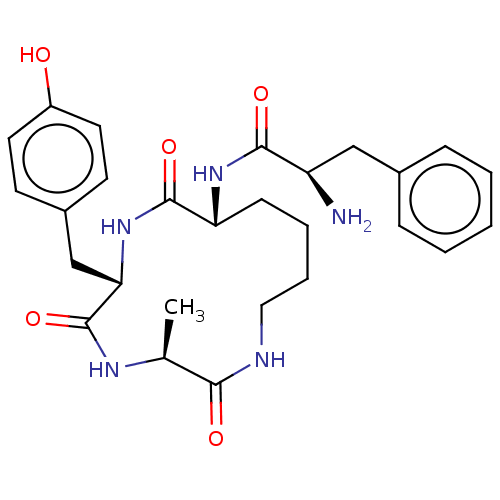 ((R)-2-Amino-N-[(R)-6-(4-hydroxy-benzyl)-3-methyl-2...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louisville Curated by ChEMBL | Assay Description In vitro mu opioid activity was determined by its ability to inhibit the electrically induced contractions of smooth muscle preparations in guinea pi... | Bioorg Med Chem Lett 9: 3441-6 (2000) BindingDB Entry DOI: 10.7270/Q2P55MQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50083733 (2-Amino-N-[(S)-6-(4-hydroxy-benzyl)-3-(1H-indol-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louisville Curated by ChEMBL | Assay Description In vitro delta opioid activity was determined by its ability to inhibit the electrically induced contractions of smooth muscle preparations in mouse ... | Bioorg Med Chem Lett 9: 3441-6 (2000) BindingDB Entry DOI: 10.7270/Q2P55MQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50083735 ((R)-2-Amino-N-[(R)-6-(4-hydroxy-benzyl)-3-methyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louisville Curated by ChEMBL | Assay Description In vitro delta opioid activity was determined by its ability to inhibit the electrically induced contractions of smooth muscle preparations in mouse ... | Bioorg Med Chem Lett 9: 3441-6 (2000) BindingDB Entry DOI: 10.7270/Q2P55MQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||