

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bile acid receptor (Homo sapiens (Human)) | BDBM50090940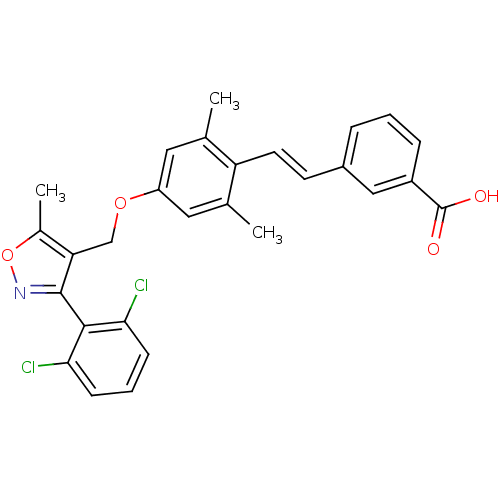 (3-(2-{4-[3-(2,6-Dichloro-phenyl)-5-methyl-isoxazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research& Development Curated by ChEMBL | Assay Description Ligand dependent recruitment of SRC1(676-700) peptide to human Farnesoid X-activated receptor by fluorescence resonance energy transfer assay | J Med Chem 43: 2971-4 (2000) BindingDB Entry DOI: 10.7270/Q2HQ40M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50090947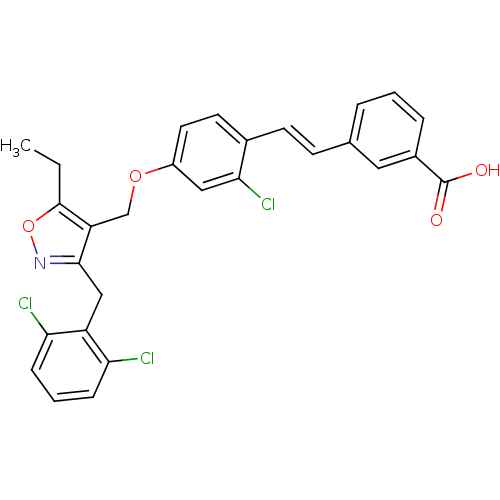 (3-(2-{2-Chloro-4-[3-(2,6-dichloro-benzyl)-5-ethyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research& Development Curated by ChEMBL | Assay Description Ligand dependent recruitment of SRC1(676-700) peptide to human Farnesoid X-activated receptor by fluorescence resonance energy transfer assay | J Med Chem 43: 2971-4 (2000) BindingDB Entry DOI: 10.7270/Q2HQ40M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50090939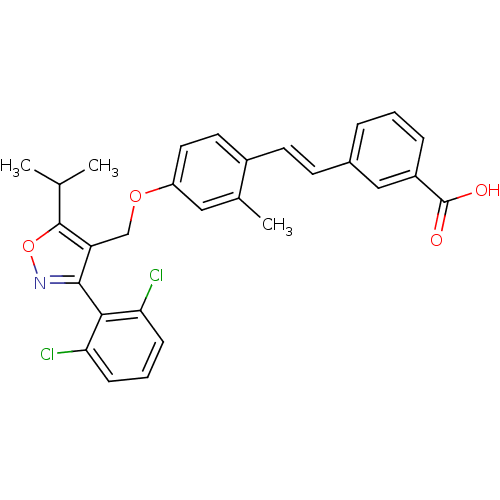 (3-(2-{4-[3-(2,6-Dichloro-phenyl)-5-isopropyl-isoxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 47 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research& Development Curated by ChEMBL | Assay Description Ligand dependent recruitment of SRC1(676-700) peptide to human Farnesoid X-activated receptor by fluorescence resonance energy transfer assay | J Med Chem 43: 2971-4 (2000) BindingDB Entry DOI: 10.7270/Q2HQ40M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM21724 (3-[(E)-2-(2-chloro-4-{[3-(2,6-dichlorophenyl)-5-(1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research& Development Curated by ChEMBL | Assay Description Ligand dependent recruitment of SRC1(676-700) peptide to human Farnesoid X-activated receptor by fluorescence resonance energy transfer assay | J Med Chem 43: 2971-4 (2000) BindingDB Entry DOI: 10.7270/Q2HQ40M3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50090945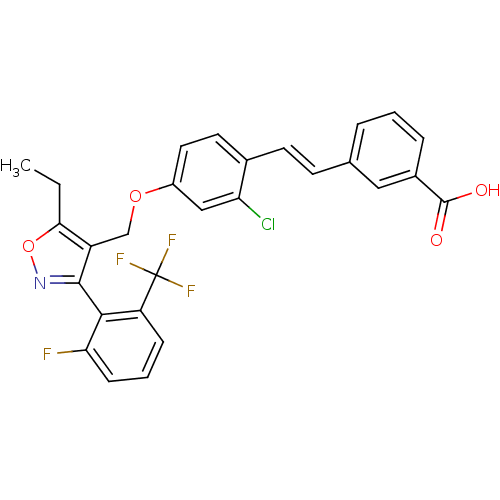 (3-(2-{2-Chloro-4-[5-ethyl-3-(2-fluoro-6-trifluorom...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 54 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research& Development Curated by ChEMBL | Assay Description Ligand dependent recruitment of SRC1(676-700) peptide to human Farnesoid X-activated receptor by fluorescence resonance energy transfer assay | J Med Chem 43: 2971-4 (2000) BindingDB Entry DOI: 10.7270/Q2HQ40M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50090948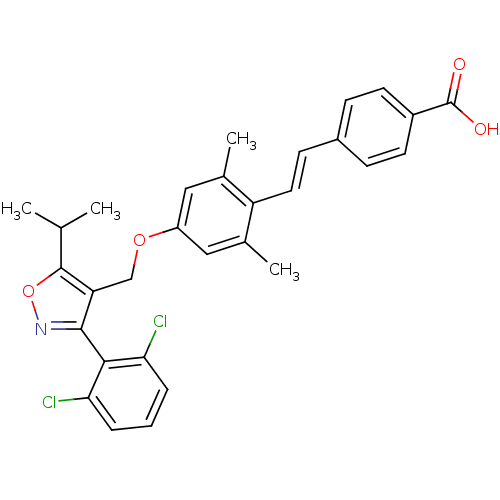 (4-(2-{4-[3-(2,6-Dichloro-phenyl)-5-isopropyl-isoxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research& Development Curated by ChEMBL | Assay Description Ligand dependent recruitment of SRC1(676-700) peptide to human Farnesoid X-activated receptor by fluorescence resonance energy transfer assay | J Med Chem 43: 2971-4 (2000) BindingDB Entry DOI: 10.7270/Q2HQ40M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50090941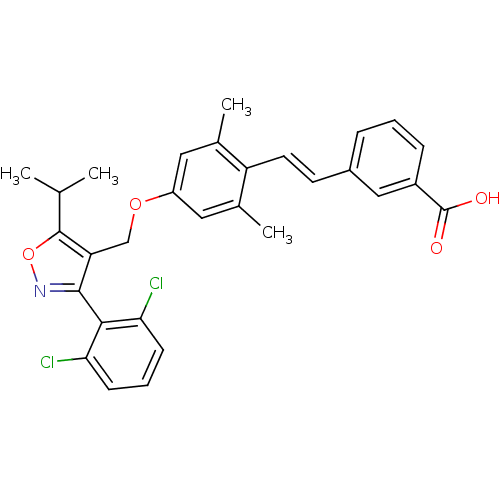 (3-(2-{4-[3-(2,6-Dichloro-phenyl)-5-isopropyl-isoxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research& Development Curated by ChEMBL | Assay Description Ligand dependent recruitment of SRC1(676-700) peptide to human Farnesoid X-activated receptor by fluorescence resonance energy transfer assay | J Med Chem 43: 2971-4 (2000) BindingDB Entry DOI: 10.7270/Q2HQ40M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50090943 (3-(2-{4-[3-(2-Bromo-6-chloro-phenyl)-5-isopropyl-i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research& Development Curated by ChEMBL | Assay Description Ligand dependent recruitment of SRC1(676-700) peptide to human Farnesoid X-activated receptor by fluorescence resonance energy transfer assay | J Med Chem 43: 2971-4 (2000) BindingDB Entry DOI: 10.7270/Q2HQ40M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50090942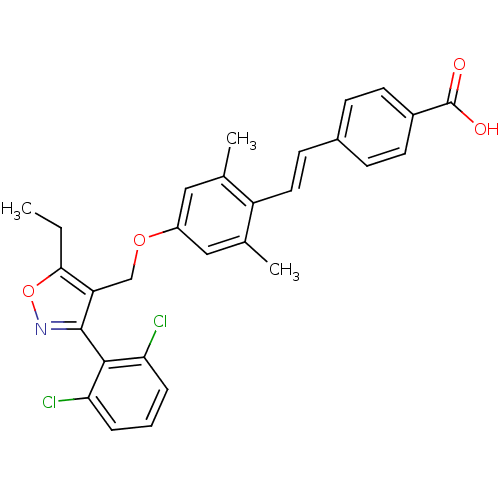 (4-(2-{4-[3-(2,6-Dichloro-phenyl)-5-ethyl-isoxazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research& Development Curated by ChEMBL | Assay Description Ligand dependent recruitment of SRC1(676-700) peptide to human Farnesoid X-activated receptor by fluorescence resonance energy transfer assay | J Med Chem 43: 2971-4 (2000) BindingDB Entry DOI: 10.7270/Q2HQ40M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50090944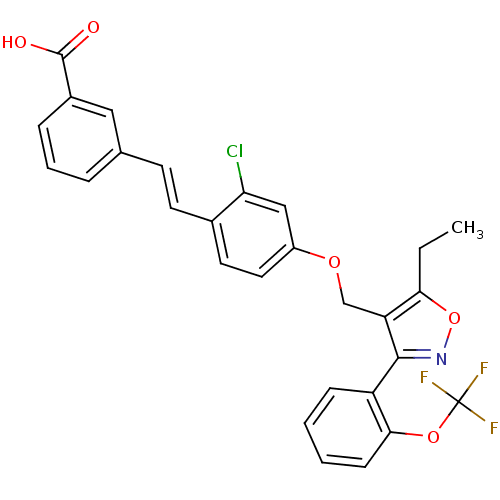 (3-(2-{2-Chloro-4-[5-ethyl-3-(2-trifluoromethoxy-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research& Development Curated by ChEMBL | Assay Description Ligand dependent recruitment of SRC1(676-700) peptide to human Farnesoid X-activated receptor by fluorescence resonance energy transfer assay | J Med Chem 43: 2971-4 (2000) BindingDB Entry DOI: 10.7270/Q2HQ40M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM21674 ((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research& Development Curated by ChEMBL | Assay Description Ligand dependent recruitment of SRC1(676-700) peptide to human Farnesoid X-activated receptor by fluorescence resonance energy transfer assay | J Med Chem 43: 2971-4 (2000) BindingDB Entry DOI: 10.7270/Q2HQ40M3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||