

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10959 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10961 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10977 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10973 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10709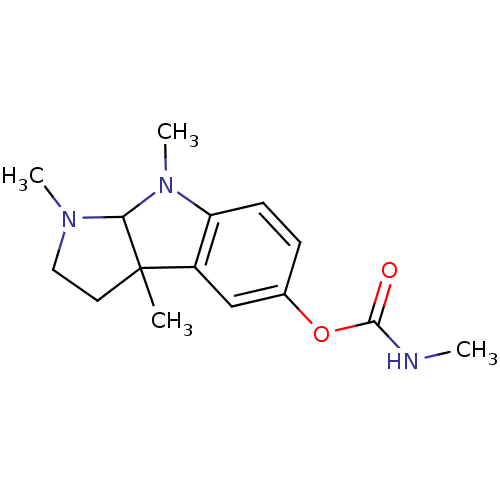 (1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo[2,3-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10958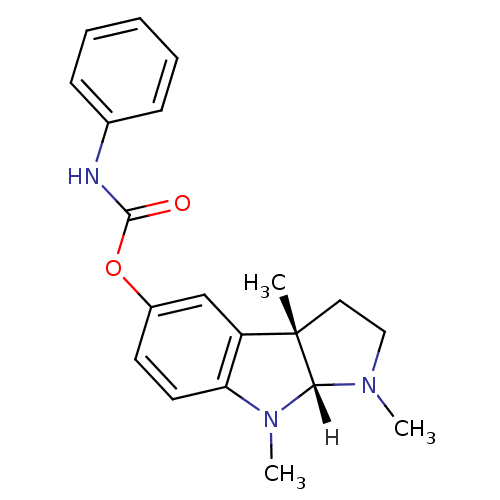 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10976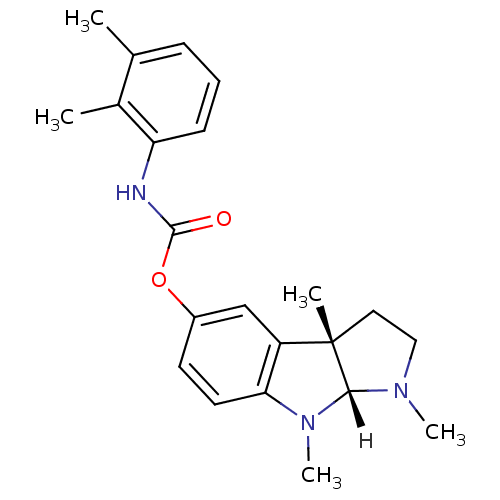 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10978 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10709 (1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo[2,3-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10974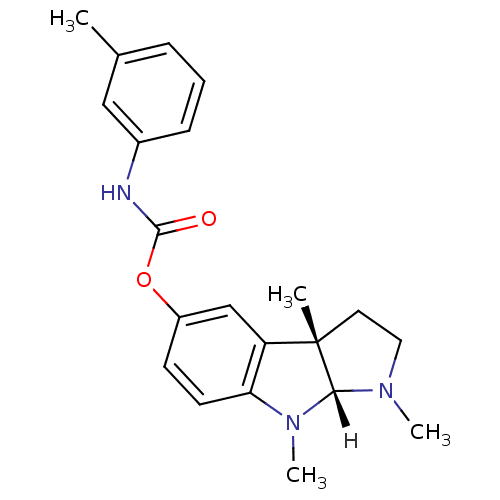 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10981 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10960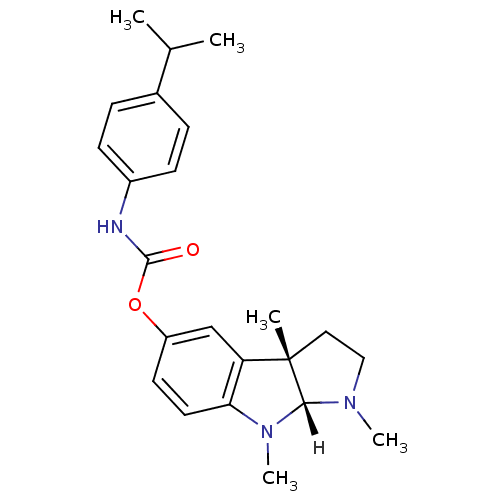 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10981 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10982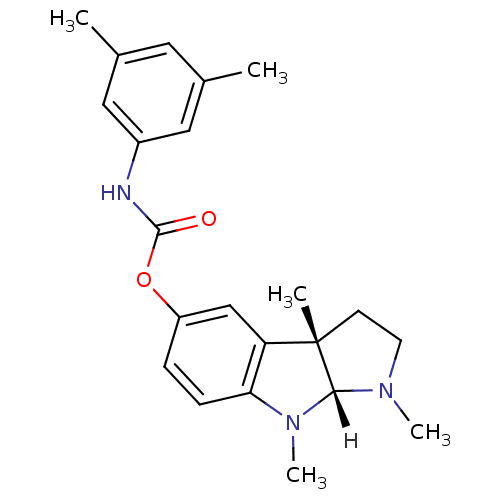 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10975 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10974 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10976 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10984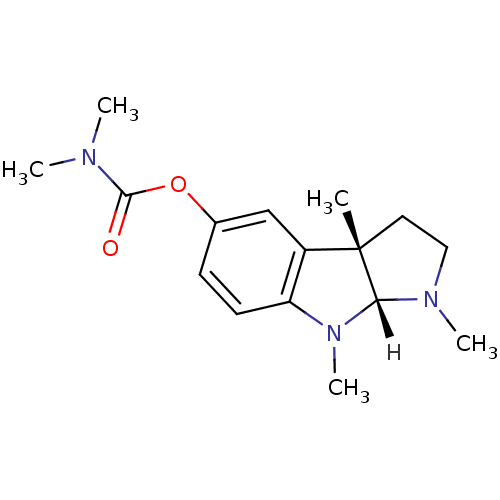 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10975 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10987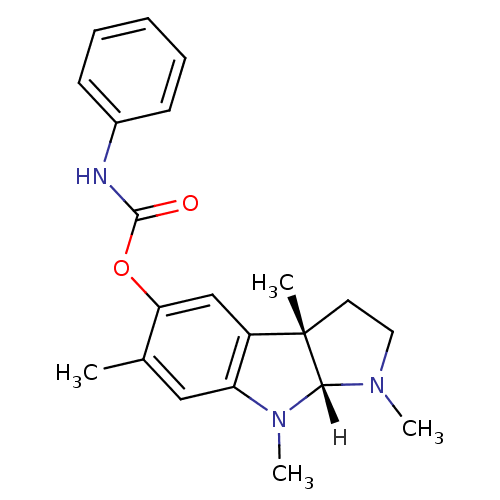 ((3aS,8aR)-1,3a,6,8-tetramethyl-1,2,3,3a,8,8a-hexah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10979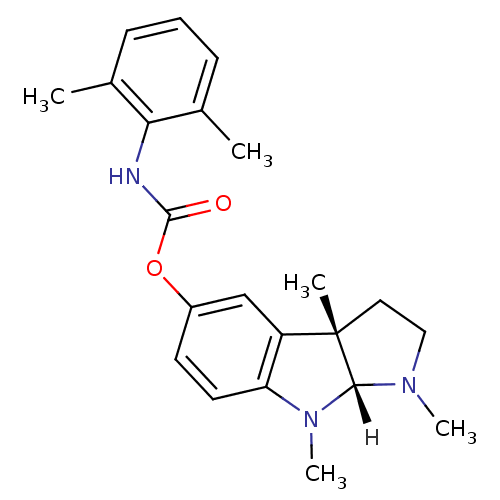 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10984 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10978 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10973 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10985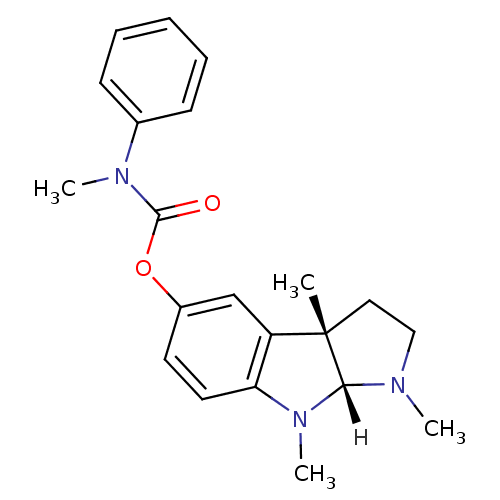 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10960 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10979 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 785 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10982 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 798 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10980 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10983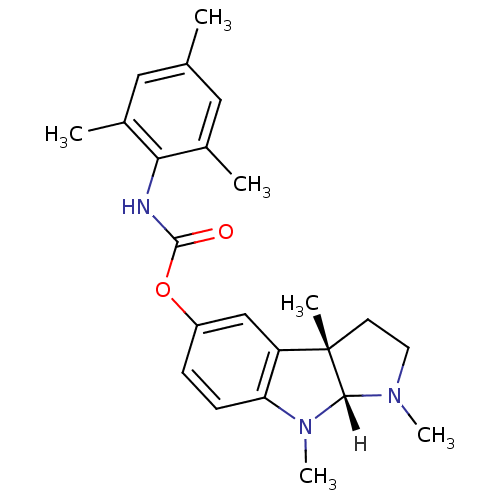 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10980 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10958 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10977 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10959 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10988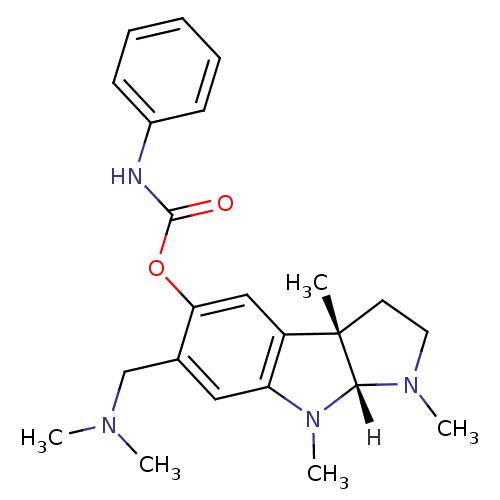 ((3aS,8aR)-6-[(dimethylamino)methyl]-1,3a,8-trimeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10961 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10983 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10988 ((3aS,8aR)-6-[(dimethylamino)methyl]-1,3a,8-trimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10987 ((3aS,8aR)-1,3a,6,8-tetramethyl-1,2,3,3a,8,8a-hexah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10986 ((3aS,8aR)-1,3a,6,8-tetramethyl-1,2,3,3a,8,8a-hexah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10985 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10986 ((3aS,8aR)-1,3a,6,8-tetramethyl-1,2,3,3a,8,8a-hexah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 4062-71 (2001) Article DOI: 10.1021/jm010080x BindingDB Entry DOI: 10.7270/Q2XS5SNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||