 Found 12 hits of Enzyme Inhibition Constant Data
Found 12 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50004907
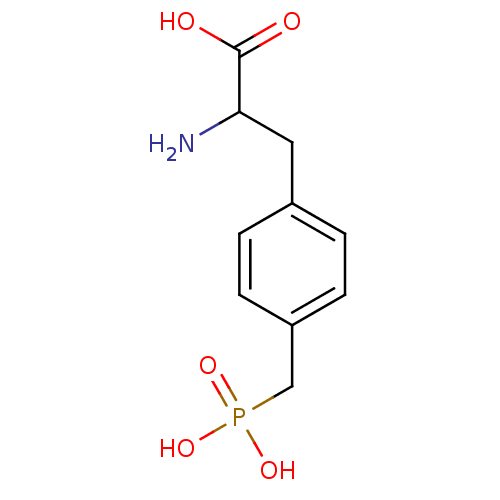
(2-Amino-3-(4-phosphonomethyl-phenyl)-propionic aci...)Show InChI InChI=1S/C10H14NO5P/c11-9(10(12)13)5-7-1-3-8(4-2-7)6-17(14,15)16/h1-4,9H,5-6,11H2,(H,12,13)(H2,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Syk C-terminal SH2 domain in in-silico screening |
J Med Chem 44: 4737-40 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DPF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50107899

(2-[4-(4-{2-[(5-Carboxy-pentyl)-(3-cyclohexyl-propy...)Show SMILES OC(=O)CCCCCN(CCCC1CCCCC1)S(=O)(=O)\C=C\c1ccc(NC(=O)[C@H]2CC[C@@H](CC2)C(C(O)=O)C(O)=O)cc1 |wU:30.30,wD:33.37,(-.42,-5.98,;.35,-4.65,;-.42,-3.32,;1.89,-4.65,;2.66,-5.98,;4.2,-5.98,;4.97,-7.33,;6.51,-7.33,;7.28,-8.66,;6.51,-9.99,;4.97,-9.99,;4.2,-11.33,;2.66,-11.33,;1.87,-12.65,;.33,-12.65,;-.44,-11.33,;.35,-9.99,;1.87,-9.99,;8.82,-8.66,;8.8,-10.2,;8.8,-7.12,;10.36,-8.64,;11.11,-7.29,;12.65,-7.28,;13.39,-5.94,;14.95,-5.91,;15.73,-7.25,;17.27,-7.23,;18.04,-5.88,;17.24,-4.57,;19.58,-5.88,;20.32,-4.53,;21.86,-4.53,;22.65,-5.85,;21.89,-7.2,;20.35,-7.2,;24.19,-5.85,;24.96,-4.5,;26.5,-4.5,;24.17,-3.19,;24.97,-7.17,;26.51,-7.17,;24.2,-8.51,;14.98,-8.58,;13.42,-8.6,)| Show InChI InChI=1S/C33H48N2O9S/c36-29(37)11-5-2-6-21-35(22-7-10-24-8-3-1-4-9-24)45(43,44)23-20-25-12-18-28(19-13-25)34-31(38)27-16-14-26(15-17-27)30(32(39)40)33(41)42/h12-13,18-20,23-24,26-27,30H,1-11,14-17,21-22H2,(H,34,38)(H,36,37)(H,39,40)(H,41,42)/b23-20+/t26-,27- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Syk C-terminal SH2. |
J Med Chem 44: 4737-40 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DPF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50107897
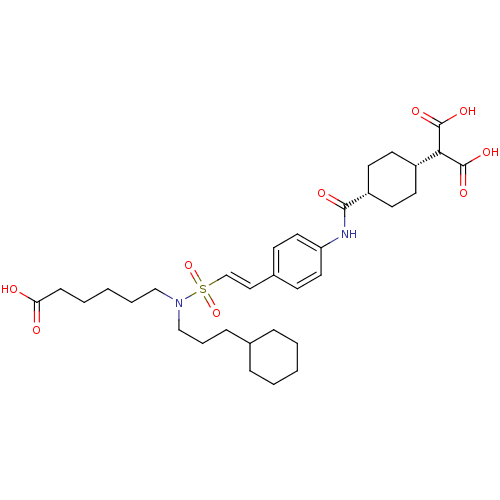
(2-[4-(4-{2-[(5-Carboxy-pentyl)-(3-cyclohexyl-propy...)Show SMILES OC(=O)CCCCCN(CCCC1CCCCC1)S(=O)(=O)\C=C\c1ccc(NC(=O)[C@@H]2CC[C@@H](CC2)C(C(O)=O)C(O)=O)cc1 |wU:33.37,30.30,(-.42,-5.98,;.35,-4.65,;-.42,-3.32,;1.89,-4.65,;2.66,-5.98,;4.2,-5.98,;4.97,-7.33,;6.51,-7.33,;7.28,-8.66,;6.51,-9.99,;4.97,-9.99,;4.2,-11.33,;2.66,-11.33,;1.87,-12.65,;.33,-12.65,;-.44,-11.33,;.35,-9.99,;1.87,-9.99,;8.82,-8.66,;8.8,-10.2,;8.8,-7.12,;10.36,-8.64,;11.11,-7.29,;12.65,-7.28,;13.39,-5.94,;14.95,-5.91,;15.73,-7.25,;17.27,-7.23,;18.04,-5.88,;17.24,-4.57,;19.58,-5.88,;20.35,-7.2,;21.89,-7.2,;22.65,-5.85,;21.86,-4.53,;20.32,-4.53,;24.19,-5.85,;24.96,-4.5,;26.5,-4.5,;24.17,-3.19,;24.97,-7.17,;26.51,-7.17,;24.2,-8.51,;14.98,-8.58,;13.42,-8.6,)| Show InChI InChI=1S/C33H48N2O9S/c36-29(37)11-5-2-6-21-35(22-7-10-24-8-3-1-4-9-24)45(43,44)23-20-25-12-18-28(19-13-25)34-31(38)27-16-14-26(15-17-27)30(32(39)40)33(41)42/h12-13,18-20,23-24,26-27,30H,1-11,14-17,21-22H2,(H,34,38)(H,36,37)(H,39,40)(H,41,42)/b23-20+/t26-,27+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Syk C-terminal SH2. |
J Med Chem 44: 4737-40 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DPF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50107901
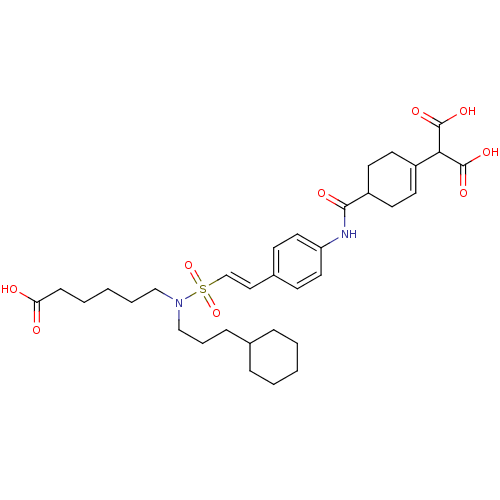
(2-[4-(4-{2-[(5-Carboxy-pentyl)-(3-cyclohexyl-propy...)Show SMILES OC(=O)CCCCCN(CCCC1CCCCC1)S(=O)(=O)\C=C\c1ccc(NC(=O)C2CCC(=CC2)C(C(O)=O)C(O)=O)cc1 |c:34| Show InChI InChI=1S/C33H46N2O9S/c36-29(37)11-5-2-6-21-35(22-7-10-24-8-3-1-4-9-24)45(43,44)23-20-25-12-18-28(19-13-25)34-31(38)27-16-14-26(15-17-27)30(32(39)40)33(41)42/h12-14,18-20,23-24,27,30H,1-11,15-17,21-22H2,(H,34,38)(H,36,37)(H,39,40)(H,41,42)/b23-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Syk C-terminal SH2. |
J Med Chem 44: 4737-40 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DPF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50107900

(2-[4-(4-{2-[(5-Carboxy-pentyl)-(5-methyl-hexyl)-su...)Show SMILES CC(C)CCCCN(CCCCCC(O)=O)S(=O)(=O)\C=C\c1ccc(NC(=O)[C@@H]2CC[C@@H](CC2)C(C(O)=O)C(O)=O)cc1 |wU:31.34,28.27,(.65,-11.61,;2.19,-11.61,;2.96,-12.94,;2.96,-10.28,;4.5,-10.28,;5.27,-8.93,;6.81,-8.93,;7.58,-7.61,;6.81,-6.27,;5.27,-6.27,;4.5,-4.94,;2.96,-4.94,;2.19,-3.6,;.65,-3.6,;-.12,-4.94,;-.12,-2.28,;9.12,-7.61,;9.12,-9.15,;9.12,-6.07,;10.66,-7.58,;11.42,-6.23,;12.96,-6.22,;13.71,-4.89,;15.25,-4.88,;16.05,-6.2,;17.59,-6.17,;18.35,-4.84,;17.56,-3.51,;19.89,-4.82,;20.66,-6.16,;22.2,-6.14,;22.95,-4.81,;22.18,-3.48,;20.64,-3.48,;24.49,-4.79,;25.26,-3.46,;26.8,-3.44,;24.49,-2.13,;25.28,-6.13,;26.82,-6.13,;24.52,-7.48,;15.28,-7.54,;13.74,-7.55,)| Show InChI InChI=1S/C31H46N2O9S/c1-22(2)8-5-7-20-33(19-6-3-4-9-27(34)35)43(41,42)21-18-23-10-16-26(17-11-23)32-29(36)25-14-12-24(13-15-25)28(30(37)38)31(39)40/h10-11,16-18,21-22,24-25,28H,3-9,12-15,19-20H2,1-2H3,(H,32,36)(H,34,35)(H,37,38)(H,39,40)/b21-18+/t24-,25+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Syk C-terminal SH2. |
J Med Chem 44: 4737-40 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DPF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50107902

(2-[4-(4-{2-[(5-Carboxy-pentyl)-(5-methyl-hexyl)-su...)Show SMILES CC(C)CCCCN(CCCCCC(O)=O)S(=O)(=O)\C=C\c1ccc(NC(=O)[C@H]2CC[C@@H](CC2)C(C(O)=O)C(O)=O)cc1 |wU:28.27,wD:31.34,(.65,-11.61,;2.19,-11.61,;2.96,-12.94,;2.96,-10.28,;4.5,-10.28,;5.27,-8.93,;6.81,-8.93,;7.58,-7.61,;6.81,-6.27,;5.27,-6.27,;4.5,-4.94,;2.96,-4.94,;2.19,-3.6,;.65,-3.6,;-.12,-4.94,;-.12,-2.28,;9.12,-7.61,;9.12,-9.15,;9.12,-6.07,;10.66,-7.58,;11.42,-6.23,;12.96,-6.22,;13.71,-4.89,;15.25,-4.88,;16.05,-6.2,;17.59,-6.17,;18.35,-4.84,;17.56,-3.51,;19.89,-4.82,;20.64,-3.48,;22.18,-3.48,;22.95,-4.81,;22.2,-6.14,;20.66,-6.16,;24.49,-4.79,;25.26,-3.46,;26.8,-3.44,;24.49,-2.13,;25.28,-6.13,;26.82,-6.13,;24.52,-7.48,;15.28,-7.54,;13.74,-7.55,)| Show InChI InChI=1S/C31H46N2O9S/c1-22(2)8-5-7-20-33(19-6-3-4-9-27(34)35)43(41,42)21-18-23-10-16-26(17-11-23)32-29(36)25-14-12-24(13-15-25)28(30(37)38)31(39)40/h10-11,16-18,21-22,24-25,28H,3-9,12-15,19-20H2,1-2H3,(H,32,36)(H,34,35)(H,37,38)(H,39,40)/b21-18+/t24-,25- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Syk C-terminal SH2. |
J Med Chem 44: 4737-40 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DPF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50107898
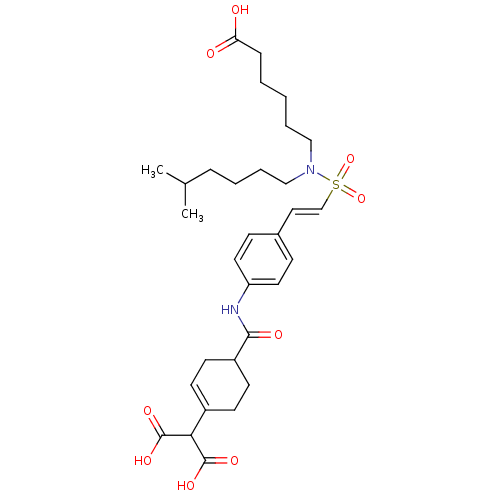
(2-[4-(4-{2-[(5-Carboxy-pentyl)-(5-methyl-hexyl)-su...)Show SMILES CC(C)CCCCN(CCCCCC(O)=O)S(=O)(=O)\C=C\c1ccc(NC(=O)C2CCC(=CC2)C(C(O)=O)C(O)=O)cc1 |c:31| Show InChI InChI=1S/C31H44N2O9S/c1-22(2)8-5-7-20-33(19-6-3-4-9-27(34)35)43(41,42)21-18-23-10-16-26(17-11-23)32-29(36)25-14-12-24(13-15-25)28(30(37)38)31(39)40/h10-12,16-18,21-22,25,28H,3-9,13-15,19-20H2,1-2H3,(H,32,36)(H,34,35)(H,37,38)(H,39,40)/b21-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Syk C-terminal SH2. |
J Med Chem 44: 4737-40 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DPF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50107895

(2-(4-{4-[2-(5-Carboxy-pentylsulfamoyl)-vinyl]-phen...)Show SMILES OC(=O)CCCCCNS(=O)(=O)\C=C\c1ccc(NC(=O)[C@H]2CCC(CC2)C(C(O)=O)C(O)=O)cc1 |wU:21.20,(-.12,-6.65,;.65,-5.3,;-.12,-3.99,;2.19,-5.3,;2.96,-6.65,;4.5,-6.65,;5.27,-7.99,;6.81,-7.99,;7.58,-9.31,;9.12,-9.31,;9.12,-7.77,;9.12,-10.85,;10.66,-9.3,;11.42,-7.96,;12.96,-7.94,;13.71,-6.59,;15.25,-6.59,;16.05,-7.9,;17.59,-7.89,;18.35,-6.56,;17.56,-5.23,;19.89,-6.54,;20.64,-5.2,;22.18,-5.2,;22.95,-6.52,;22.2,-7.86,;20.66,-7.87,;24.49,-6.51,;25.26,-5.18,;26.8,-5.17,;24.49,-3.85,;25.28,-7.84,;26.82,-7.84,;24.52,-9.19,;15.28,-9.25,;13.74,-9.25,)| Show InChI InChI=1S/C24H32N2O9S/c27-20(28)4-2-1-3-14-25-36(34,35)15-13-16-5-11-19(12-6-16)26-22(29)18-9-7-17(8-10-18)21(23(30)31)24(32)33/h5-6,11-13,15,17-18,21,25H,1-4,7-10,14H2,(H,26,29)(H,27,28)(H,30,31)(H,32,33)/b15-13+/t17?,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Syk C-terminal SH2. |
J Med Chem 44: 4737-40 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DPF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50107903
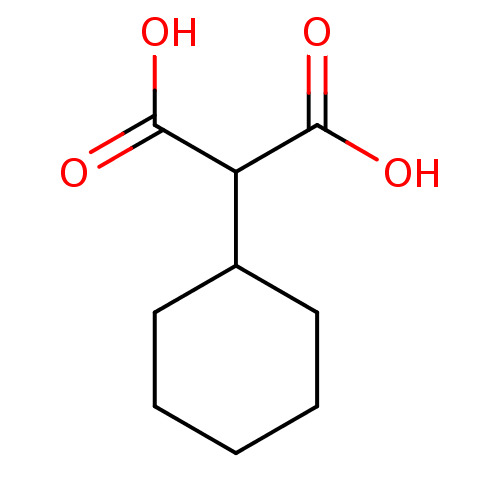
(2-Cyclohexyl-malonic acid | CHEMBL142870)Show InChI InChI=1S/C9H14O4/c10-8(11)7(9(12)13)6-4-2-1-3-5-6/h6-7H,1-5H2,(H,10,11)(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Syk C-terminal SH2. |
J Med Chem 44: 4737-40 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DPF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50107894
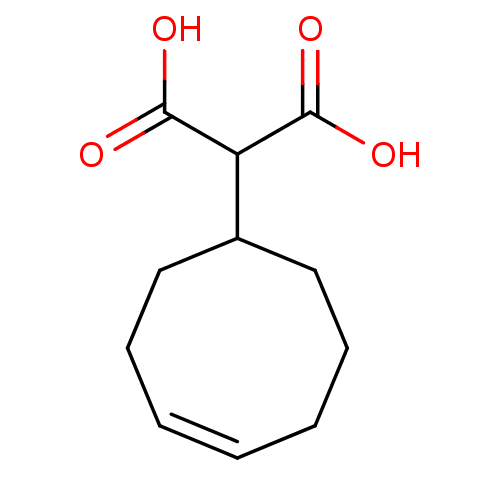
(2-Cyclooct-4-enyl-malonic acid | CHEMBL357125)Show InChI InChI=1S/C11H16O4/c12-10(13)9(11(14)15)8-6-4-2-1-3-5-7-8/h1-2,8-9H,3-7H2,(H,12,13)(H,14,15)/b2-1- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 7.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Syk C-terminal SH2. |
J Med Chem 44: 4737-40 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DPF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50107896

(6-(2-Phenyl-ethenesulfonylamino)-hexanoic acid | C...)Show InChI InChI=1S/C14H19NO4S/c16-14(17)9-5-2-6-11-15-20(18,19)12-10-13-7-3-1-4-8-13/h1,3-4,7-8,10,12,15H,2,5-6,9,11H2,(H,16,17)/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 8.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Syk C-terminal SH2 (at 10 mM) |
J Med Chem 44: 4737-40 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DPF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM14676

(2-phenylpropanedioic acid | CHEMBL78794 | Fragment...)Show InChI InChI=1S/C9H8O4/c10-8(11)7(9(12)13)6-4-2-1-3-5-6/h1-5,7H,(H,10,11)(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Waseda University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Syk C-terminal SH2. |
J Med Chem 44: 4737-40 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DPF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data