 Found 24 hits of Enzyme Inhibition Constant Data
Found 24 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50108364

(CHEMBL293098 | N-(5-Bromo-3-chloro-pyridin-2-yl)-g...)Show InChI InChI=1S/C6H6BrClN4/c7-3-1-4(8)5(11-2-3)12-6(9)10/h1-2H,(H4,9,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50108362

(CHEMBL54357 | N-(5-Bromo-pyridin-2-yl)-guanidine)Show InChI InChI=1S/C6H7BrN4/c7-4-1-2-5(10-3-4)11-6(8)9/h1-3H,(H4,8,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50108360
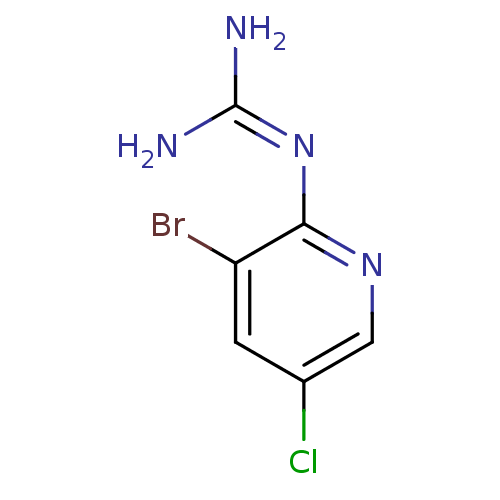
(CHEMBL55125 | N-(3-Bromo-5-chloro-pyridin-2-yl)-gu...)Show InChI InChI=1S/C6H6BrClN4/c7-4-1-3(8)2-11-5(4)12-6(9)10/h1-2H,(H4,9,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 4.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50108354
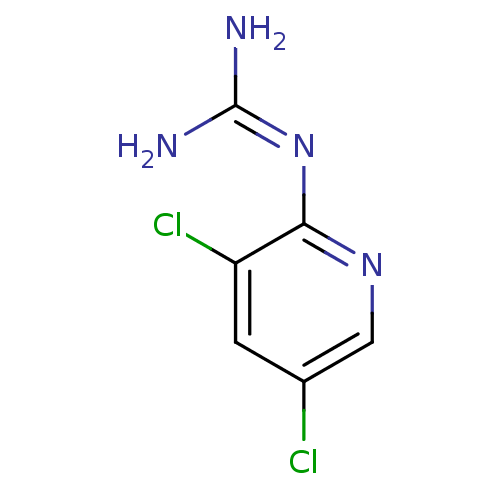
(CHEMBL292900 | N-(3,5-Dichloro-pyridin-2-yl)-guani...)Show InChI InChI=1S/C6H6Cl2N4/c7-3-1-4(8)5(11-2-3)12-6(9)10/h1-2H,(H4,9,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50053590
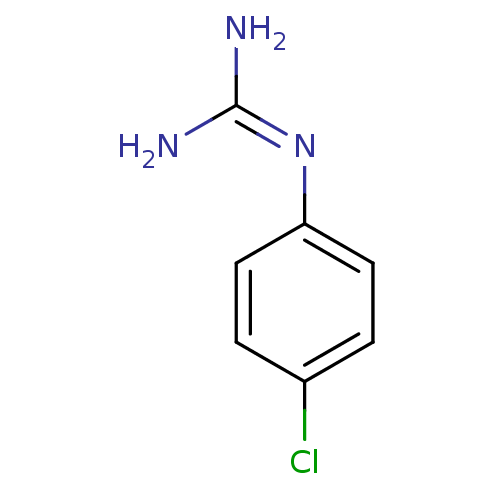
(1N-amino(immino)methyl-4-chloroaniline | CHEMBL410...)Show InChI InChI=1S/C7H8ClN3/c8-5-1-3-6(4-2-5)11-7(9)10/h1-4H,(H4,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50108355
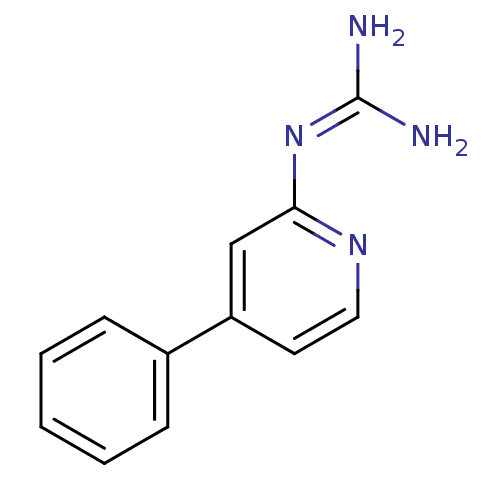
(CHEMBL54467 | N-(4-Phenyl-pyridin-2-yl)-guanidine)Show InChI InChI=1S/C12H12N4/c13-12(14)16-11-8-10(6-7-15-11)9-4-2-1-3-5-9/h1-8H,(H4,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50108352

(CHEMBL55808 | N-(3,5-Dichloro-4-methyl-pyridin-2-y...)Show SMILES [#6]-c1c(Cl)cnc(\[#7]=[#6](\[#7])-[#7])c1Cl Show InChI InChI=1S/C7H8Cl2N4/c1-3-4(8)2-12-6(5(3)9)13-7(10)11/h2H,1H3,(H4,10,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50108363

(CHEMBL298989 | N-(5-Chloro-pyridin-2-yl)-guanidine)Show InChI InChI=1S/C6H7ClN4/c7-4-1-2-5(10-3-4)11-6(8)9/h1-3H,(H4,8,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50108353
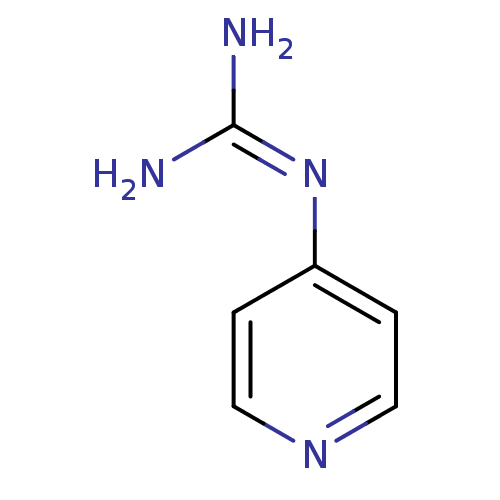
(CHEMBL298550 | N-Pyridin-4-yl-guanidine)Show InChI InChI=1S/C6H8N4/c7-6(8)10-5-1-3-9-4-2-5/h1-4H,(H4,7,8,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Ability to inhibit human plasmin using Chromozym-PL as substrate |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50053618
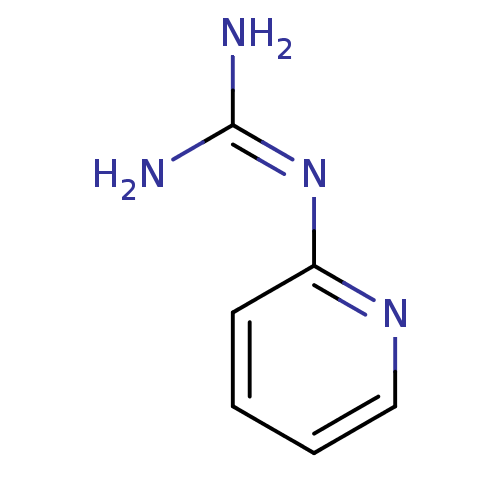
(CHEMBL128047 | N-Pyridin-2-yl-guanidine)Show InChI InChI=1S/C6H8N4/c7-6(8)10-5-3-1-2-4-9-5/h1-4H,(H4,7,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 2.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50108365

(CHEMBL53031 | N-(5-Methyl-pyridin-2-yl)-guanidine)Show InChI InChI=1S/C7H10N4/c1-5-2-3-6(10-4-5)11-7(8)9/h2-4H,1H3,(H4,8,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50108358

(CHEMBL52361 | N-(3-Methyl-pyridin-2-yl)-guanidine)Show InChI InChI=1S/C7H10N4/c1-5-3-2-4-10-6(5)11-7(8)9/h2-4H,1H3,(H4,8,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50108361
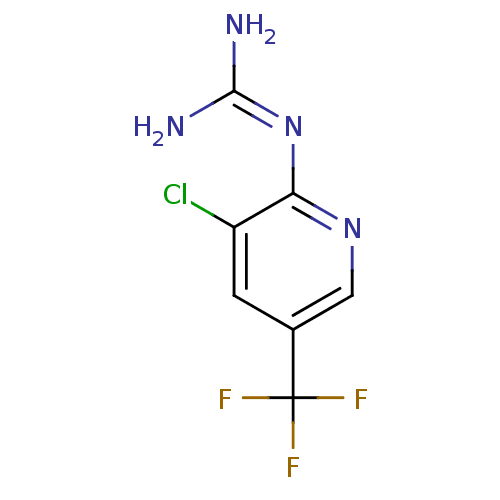
(CHEMBL53032 | N-(3-Chloro-5-trifluoromethyl-pyridi...)Show InChI InChI=1S/C7H6ClF3N4/c8-4-1-3(7(9,10)11)2-14-5(4)15-6(12)13/h1-2H,(H4,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 4.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50053590

(1N-amino(immino)methyl-4-chloroaniline | CHEMBL410...)Show InChI InChI=1S/C7H8ClN3/c8-5-1-3-6(4-2-5)11-7(9)10/h1-4H,(H4,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Ability to inhibit human plasmin using Chromozym-PL as substrate |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50108367
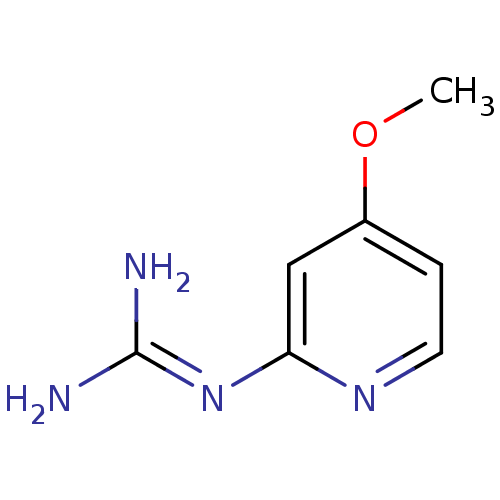
(CHEMBL55184 | N-(4-Methoxy-pyridin-2-yl)-guanidine)Show InChI InChI=1S/C7H10N4O/c1-12-5-2-3-10-6(4-5)11-7(8)9/h2-4H,1H3,(H4,8,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50108359

(CHEMBL300135 | N-(3-Methoxy-pyridin-2-yl)-guanidin...)Show InChI InChI=1S/C7H10N4O/c1-12-5-3-2-4-10-6(5)11-7(8)9/h2-4H,1H3,(H4,8,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50108366

(CHEMBL54640 | N-(4-Methyl-pyridin-2-yl)-guanidine)Show InChI InChI=1S/C7H10N4/c1-5-2-3-10-6(4-5)11-7(8)9/h2-4H,1H3,(H4,8,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 8.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50108356

(CHEMBL55051 | N-(3-Hydroxy-pyridin-2-yl)-guanidine)Show InChI InChI=1S/C6H8N4O/c7-6(8)10-5-4(11)2-1-3-9-5/h1-3,11H,(H4,7,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50108357

(CHEMBL54545 | N-(6-Methyl-pyridin-2-yl)-guanidine)Show InChI InChI=1S/C7H10N4/c1-5-3-2-4-6(10-5)11-7(8)9/h2-4H,1H3,(H4,8,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.73E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50108352

(CHEMBL55808 | N-(3,5-Dichloro-4-methyl-pyridin-2-y...)Show SMILES [#6]-c1c(Cl)cnc(\[#7]=[#6](\[#7])-[#7])c1Cl Show InChI InChI=1S/C7H8Cl2N4/c1-3-4(8)2-12-6(5(3)9)13-7(10)11/h2H,1H3,(H4,10,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Ability to inhibit human plasmin using Chromozym-PL as substrate |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50108359

(CHEMBL300135 | N-(3-Methoxy-pyridin-2-yl)-guanidin...)Show InChI InChI=1S/C7H10N4O/c1-12-5-3-2-4-10-6(5)11-7(8)9/h2-4H,1H3,(H4,8,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <2.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Ability to inhibit human plasmin using Chromozym-PL as substrate |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50108365

(CHEMBL53031 | N-(5-Methyl-pyridin-2-yl)-guanidine)Show InChI InChI=1S/C7H10N4/c1-5-2-3-6(10-4-5)11-7(8)9/h2-4H,1H3,(H4,8,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Ability to inhibit human tissue plasminogen activator stimulator |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50108360

(CHEMBL55125 | N-(3-Bromo-5-chloro-pyridin-2-yl)-gu...)Show InChI InChI=1S/C6H6BrClN4/c7-4-1-3(8)2-11-5(4)12-6(9)10/h1-2H,(H4,9,10,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.72E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Ability to inhibit human tissue plasminogen activator stimulator |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50108368

(CHEMBL54989 | N-Pyrimidin-2-yl-guanidine)Show InChI InChI=1S/C5H7N5/c6-4(7)10-5-8-2-1-3-9-5/h1-3H,(H4,6,7,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 2.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of HWMT human urokinase Plasminogen activator. |
Bioorg Med Chem Lett 12: 181-4 (2001)
BindingDB Entry DOI: 10.7270/Q2N8794F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data