

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Human rhinovirus B) | BDBM50112659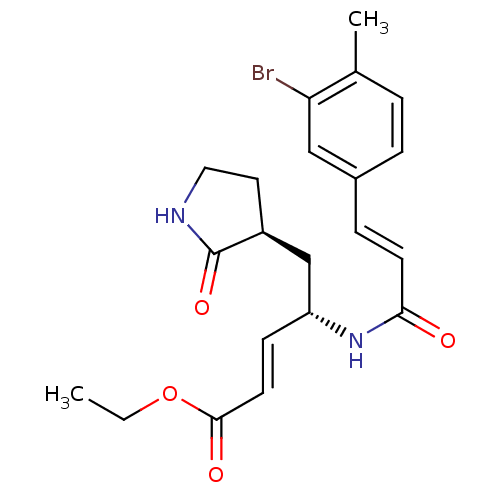 (4-[3-(3-Bromo-4-methyl-phenyl)-acryloylamino]-5-(2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Rhinovirus 14 (HRV14) Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112647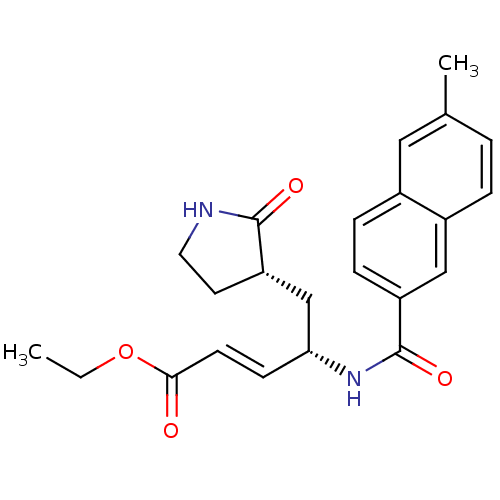 (4-[(6-Methyl-naphthalene-2-carbonyl)-amino]-5-(2-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 880 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Rhinovirus 14 (HRV14) Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112656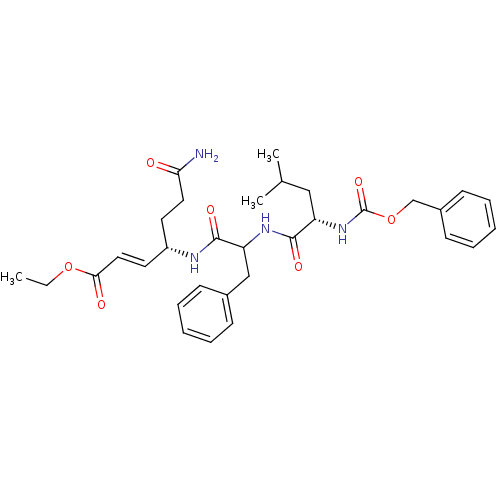 (4-[2-(2-Benzyloxycarbonylamino-4-methyl-pentanoyla...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 540 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Rhinovirus 14 (HRV14) Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112651 (4-[(6-Chloro-2H-chromene-3-carbonyl)-amino]-5-(2-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description 50% effective concentration required to inhibit Human Rhinovirus 1A Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112656 (4-[2-(2-Benzyloxycarbonylamino-4-methyl-pentanoyla...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description 50% effective concentration required to inhibit Human Rhinovirus 10 Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112655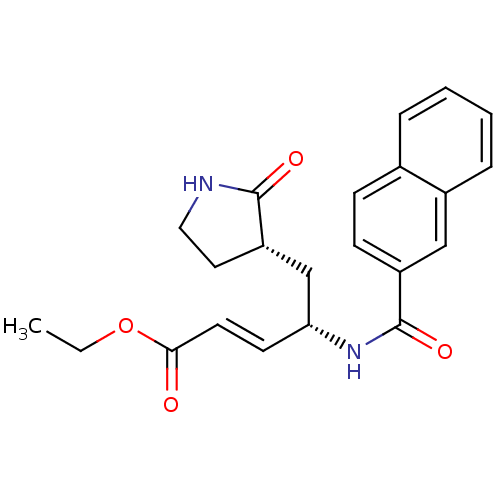 (4-[(Naphthalene-2-carbonyl)-amino]-5-(2-oxo-pyrrol...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description 50% effective concentration required to inhibit Human Rhinovirus 2 Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112648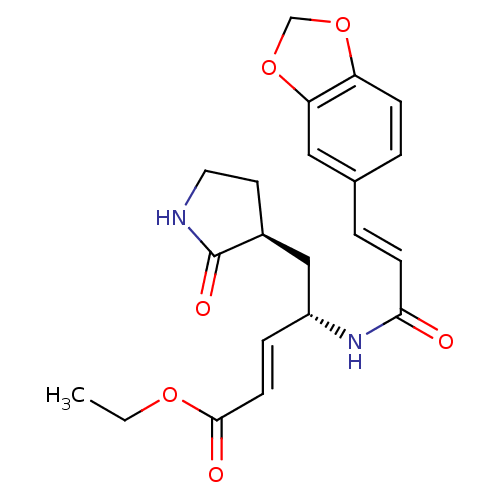 (4-(3-Benzo[1,3]dioxol-5-yl-acryloylamino)-5-(2-oxo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Rhinovirus 14 (HRV14) Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112654 (4-[(2-Methyl-5-phenyl-furan-3-carbonyl)-amino]-5-(...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Rhinovirus 14 (HRV14) Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112653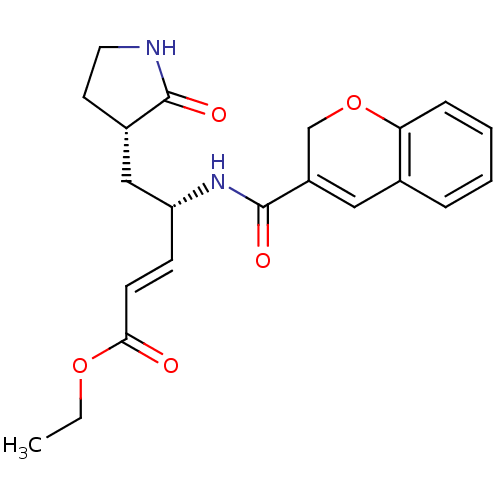 (4-[(2H-Chromene-3-carbonyl)-amino]-5-(2-oxo-pyrrol...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Rhinovirus 14 (HRV14) Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112655 (4-[(Naphthalene-2-carbonyl)-amino]-5-(2-oxo-pyrrol...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Rhinovirus 14 (HRV14) Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112650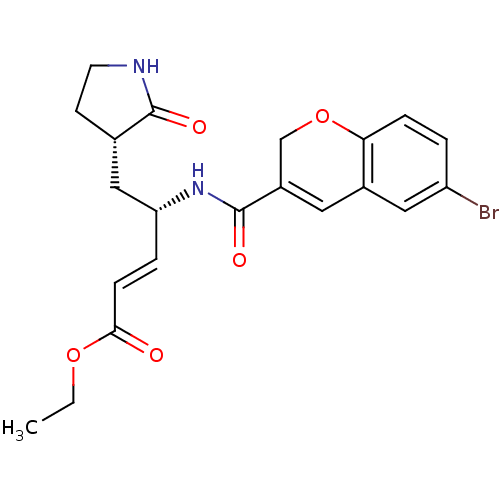 (4-[(6-Bromo-2H-chromene-3-carbonyl)-amino]-5-(2-ox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Rhinovirus 14 (HRV14) Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112656 (4-[2-(2-Benzyloxycarbonylamino-4-methyl-pentanoyla...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description 50% effective concentration required to inhibit Human Rhinovirus 89 Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112651 (4-[(6-Chloro-2H-chromene-3-carbonyl)-amino]-5-(2-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 460 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description 50% effective concentration required to inhibit Human Rhinovirus 10 Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112651 (4-[(6-Chloro-2H-chromene-3-carbonyl)-amino]-5-(2-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Rhinovirus 14 (HRV14) Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112650 (4-[(6-Bromo-2H-chromene-3-carbonyl)-amino]-5-(2-ox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 550 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description 50% effective concentration required to inhibit Human Rhinovirus 10 Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112651 (4-[(6-Chloro-2H-chromene-3-carbonyl)-amino]-5-(2-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 650 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description 50% effective concentration required to inhibit Human Rhinovirus 2 Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112656 (4-[2-(2-Benzyloxycarbonylamino-4-methyl-pentanoyla...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description 50% effective concentration required to inhibit Human Rhinovirus 2 Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112649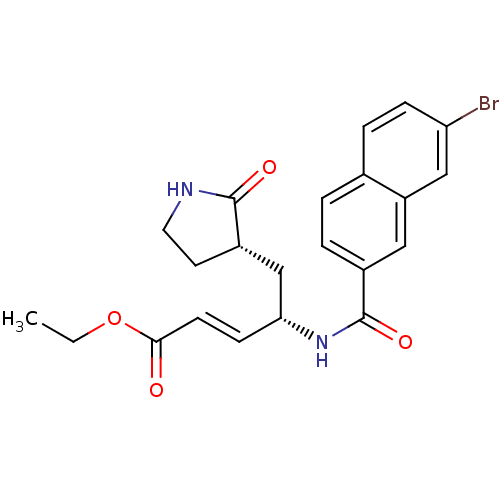 (4-[(7-Bromo-naphthalene-2-carbonyl)-amino]-5-(2-ox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Rhinovirus 14 (HRV14) Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112657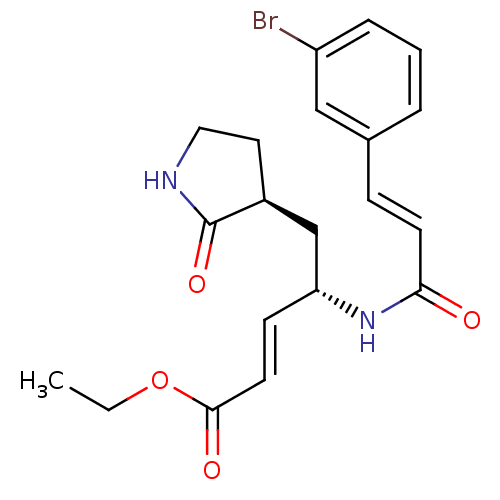 (4-[3-(3-Bromo-phenyl)-acryloylamino]-5-(2-oxo-pyrr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Rhinovirus 14 (HRV14) Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112658 (4-[3-(6-Bromo-benzo[1,3]dioxol-5-yl)-acryloylamino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 490 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Rhinovirus 14 (HRV14) Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112656 (4-[2-(2-Benzyloxycarbonylamino-4-methyl-pentanoyla...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description 50% effective concentration required to inhibit Human Rhinovirus 16 Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112652 (4-[3-(3-Bromo-4-fluoro-phenyl)-acryloylamino]-5-(2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 630 | n/a | n/a | n/a | n/a |
Pfizer Global R&D-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Rhinovirus 14 (HRV14) Protease 3C | J Med Chem 45: 2016-23 (2002) BindingDB Entry DOI: 10.7270/Q289156B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||