

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50118067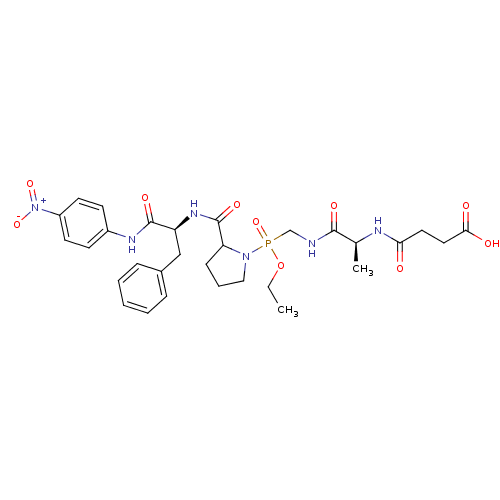 (CHEMBL126888 | N-{1-[(Ethoxy-{2-[1-(4-nitro-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement d'Ing�nierie et d'Etudes des Prot�ines Curated by ChEMBL | Assay Description Inhibitory activity against Human Cyclophilin hCyp-18 Peptidyl-prolyl isomerase by using the standard trypsin-coupled PPIase (peptidyl-prolyl isomera... | J Med Chem 45: 3928-33 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50118067 (CHEMBL126888 | N-{1-[(Ethoxy-{2-[1-(4-nitro-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement d'Ing�nierie et d'Etudes des Prot�ines Curated by ChEMBL | Assay Description Inhibitory activity against Human Cyclophilin hCyp-18 Peptidyl-prolyl isomerase by using the standard trypsin-coupled PPIase (peptidyl-prolyl isomera... | J Med Chem 45: 3928-33 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50118069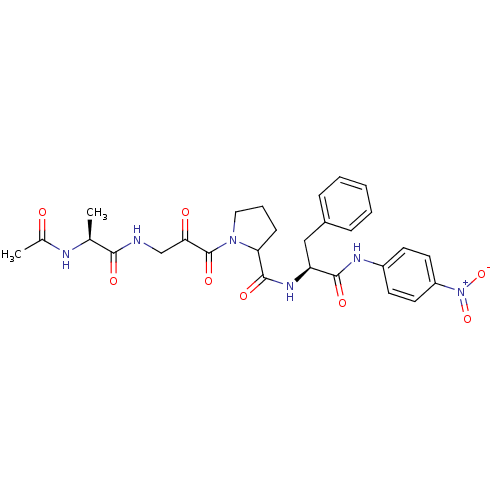 (1-[3-(2-Acetylamino-propionylamino)-2-oxo-propiony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement d'Ing�nierie et d'Etudes des Prot�ines Curated by ChEMBL | Assay Description Inhibitory activity against Human Cyclophilin hCyp-18 Peptidyl-prolyl isomerase by using the standard trypsin-coupled PPIase (peptidyl-prolyl isomera... | J Med Chem 45: 3928-33 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50087877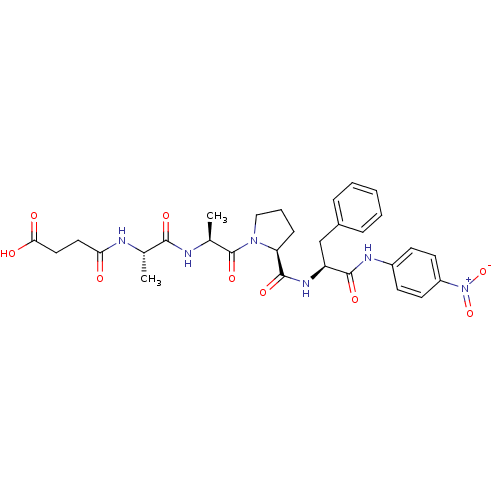 (CHEMBL298712 | N-[1-(1-Methyl-2-{2-[1-(4-nitro-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement d'Ing�nierie et d'Etudes des Prot�ines Curated by ChEMBL | Assay Description Inhibitory activity against Human Cyclophilin hCyp-18 Peptidyl-prolyl isomerase by using the standard trypsin-coupled PPIase (peptidyl-prolyl isomera... | J Med Chem 45: 3928-33 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50118068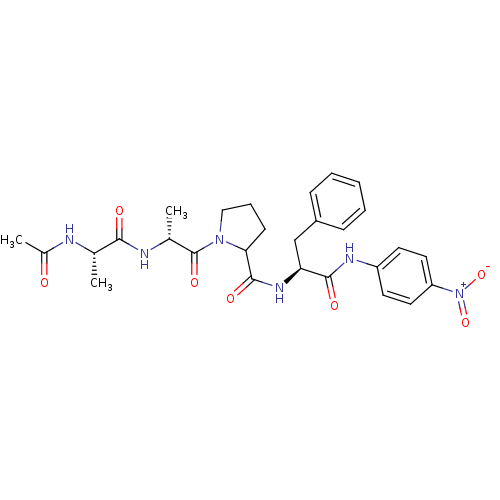 (1-[2-(2-Acetylamino-propionylamino)-propionyl]-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement d'Ing�nierie et d'Etudes des Prot�ines Curated by ChEMBL | Assay Description Inhibitory activity against Human Cyclophilin hCyp-18 Peptidyl-prolyl isomerase by using the standard trypsin-coupled PPIase (peptidyl-prolyl isomera... | J Med Chem 45: 3928-33 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50118071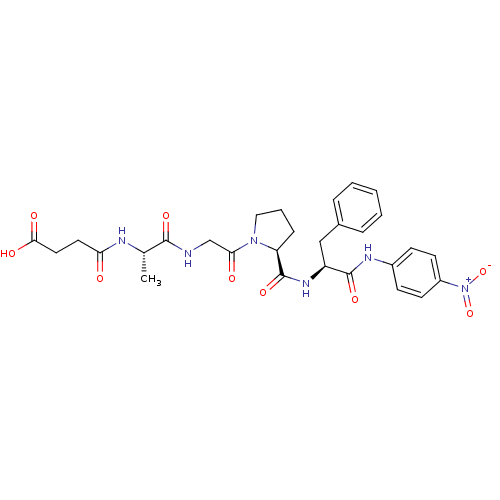 (CHEMBL125967 | N-[1-(2-{2-[1-(4-Nitro-phenylcarbam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.45E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
D�partement d'Ing�nierie et d'Etudes des Prot�ines Curated by ChEMBL | Assay Description Inhibitory activity against Human Cyclophilin hCyp-18 Peptidyl-prolyl isomerase by using the standard trypsin-coupled PPIase (peptidyl-prolyl isomera... | J Med Chem 45: 3928-33 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50118067 (CHEMBL126888 | N-{1-[(Ethoxy-{2-[1-(4-nitro-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a |
D�partement d'Ing�nierie et d'Etudes des Prot�ines Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity against Human Cyclophilin hCyp-18 Peptidyl-prolyl isomerase | J Med Chem 45: 3928-33 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50118072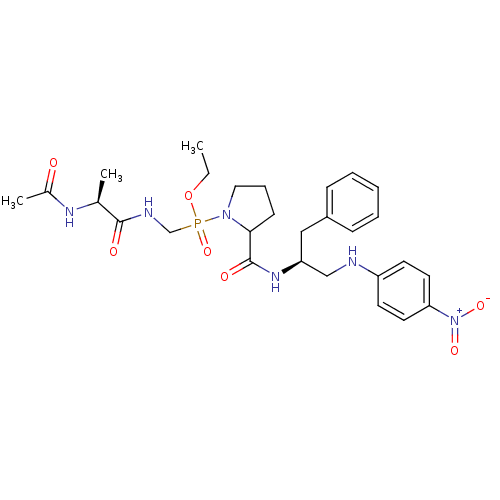 (CHEMBL126876 | [(2-Acetylamino-propionylamino)-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a |
D�partement d'Ing�nierie et d'Etudes des Prot�ines Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity against Human Cyclophilin hCyp-18 Peptidyl-prolyl isomerase | J Med Chem 45: 3928-33 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50118069 (1-[3-(2-Acetylamino-propionylamino)-2-oxo-propiony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 1.27E+5 | n/a | n/a | n/a | n/a | n/a |
D�partement d'Ing�nierie et d'Etudes des Prot�ines Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity against Human Cyclophilin hCyp-18 Peptidyl-prolyl isomerase | J Med Chem 45: 3928-33 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50118072 (CHEMBL126876 | [(2-Acetylamino-propionylamino)-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a |
D�partement d'Ing�nierie et d'Etudes des Prot�ines Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity against Human Cyclophilin hCyp-18 Peptidyl-prolyl isomerase | J Med Chem 45: 3928-33 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50118066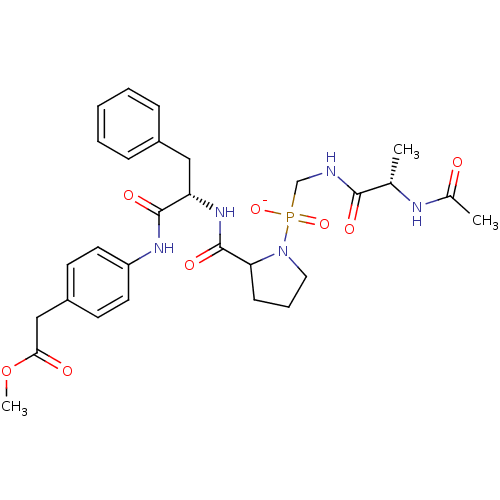 ([(2-Acetylamino-propionylamino)-methyl]-{2-[1-(4-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a |
D�partement d'Ing�nierie et d'Etudes des Prot�ines Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity against Human Cyclophilin hCyp-18 Peptidyl-prolyl isomerase | J Med Chem 45: 3928-33 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50118067 (CHEMBL126888 | N-{1-[(Ethoxy-{2-[1-(4-nitro-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a |
D�partement d'Ing�nierie et d'Etudes des Prot�ines Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity against Human Cyclophilin hCyp-18 Peptidyl-prolyl isomerase | J Med Chem 45: 3928-33 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50087877 (CHEMBL298712 | N-[1-(1-Methyl-2-{2-[1-(4-nitro-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 1.35E+5 | n/a | n/a | n/a | n/a | n/a |
D�partement d'Ing�nierie et d'Etudes des Prot�ines Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity against Human Cyclophilin hCyp-18 Peptidyl-prolyl isomerase | J Med Chem 45: 3928-33 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50118068 (1-[2-(2-Acetylamino-propionylamino)-propionyl]-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a |
D�partement d'Ing�nierie et d'Etudes des Prot�ines Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity against Human Cyclophilin hCyp-18 Peptidyl-prolyl isomerase | J Med Chem 45: 3928-33 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50118071 (CHEMBL125967 | N-[1-(2-{2-[1-(4-Nitro-phenylcarbam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a |
D�partement d'Ing�nierie et d'Etudes des Prot�ines Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity against Human Cyclophilin hCyp-18 Peptidyl-prolyl isomerase | J Med Chem 45: 3928-33 (2002) BindingDB Entry DOI: 10.7270/Q2CZ37WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||