

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50003168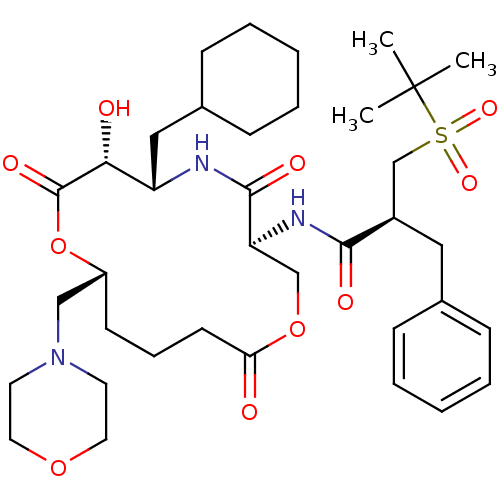 (2-Benzyl-N-(6-cyclohexylmethyl-7-hydroxy-10-morpho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003195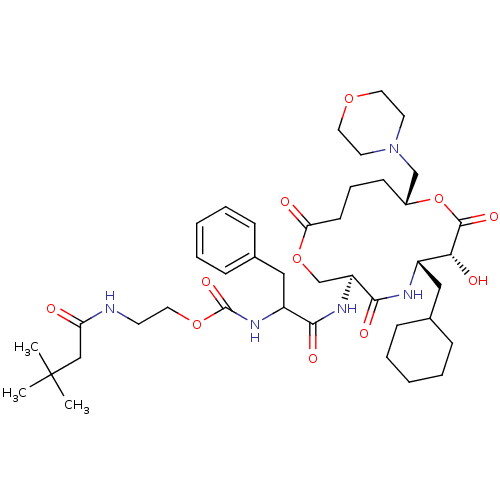 (CHEMBL126115 | [1-(6-Cyclohexylmethyl-7-hydroxy-10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003191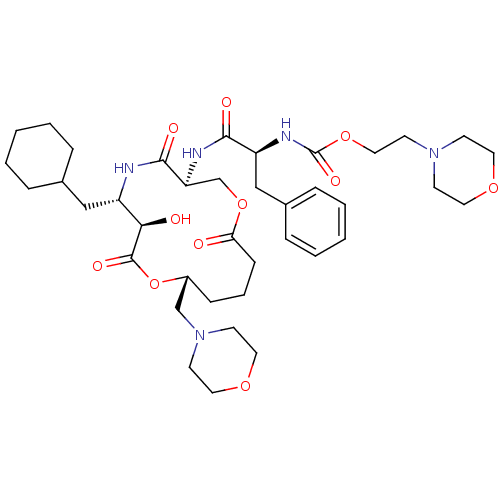 (CHEMBL338008 | [1-(6-Cyclohexylmethyl-7-hydroxy-10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003194 (CHEMBL124616 | [1-(6-Cyclohexylmethyl-7-hydroxy-10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003174 (CHEMBL339289 | {2-[1-(6-Cyclohexylmethyl-7-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003176 (2-[1-(6-Cyclohexylmethyl-7-hydroxy-10-morpholin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003165 (1N-[1-[12-cyclohexylmethyl-11-hydroxy-8-(1,4-oxazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003192 (2-(1-Aza-bicyclo[2.2.2]oct-3-ylamino)-N-(6-cyclohe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003181 ((diastereomer-1) (1-{6-Cyclohexylmethyl-7-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003181 ((diastereomer-1) (1-{6-Cyclohexylmethyl-7-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50230157 (CHEMBL3144232) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003190 ((diastereomer-1)[1-(6-Cyclohexylmethyl-7-hydroxy-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003177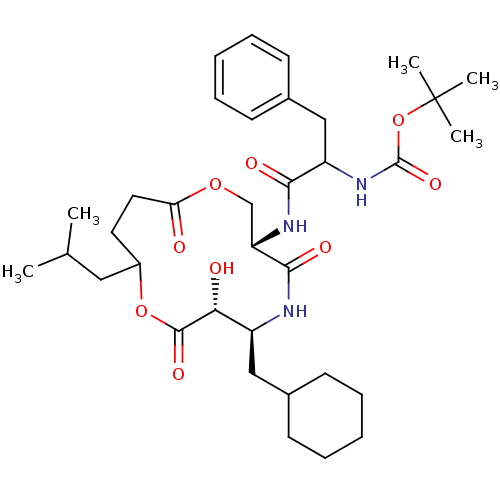 ((diastereomer-1) [1-(6-Cyclohexylmethyl-7-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003188 ((diastereomer-1) [1-(6-Cyclohexylmethyl-10-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003188 ((diastereomer-1) [1-(6-Cyclohexylmethyl-10-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003177 ((diastereomer-1) [1-(6-Cyclohexylmethyl-7-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003171 ((diastereomer-1) [1-(6-Cyclohexylmethyl-10-diethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003171 ((diastereomer-1) [1-(6-Cyclohexylmethyl-10-diethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003175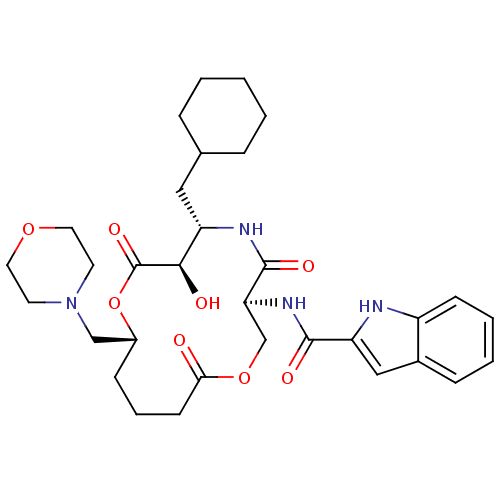 (1H-Indole-2-carboxylic acid (6-cyclohexylmethyl-7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003183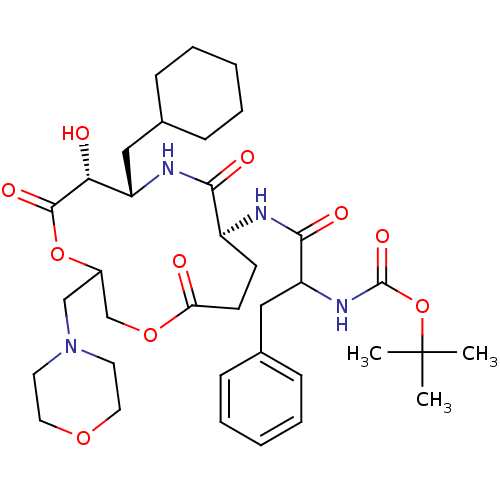 ((diastereomer-1)[1-(7-Cyclohexylmethyl-6-hydroxy-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003183 ((diastereomer-1)[1-(7-Cyclohexylmethyl-6-hydroxy-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003173 ((diastereomer-1)[1-(10-Butyl-6-cyclohexylmethyl-7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003173 ((diastereomer-1)[1-(10-Butyl-6-cyclohexylmethyl-7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003166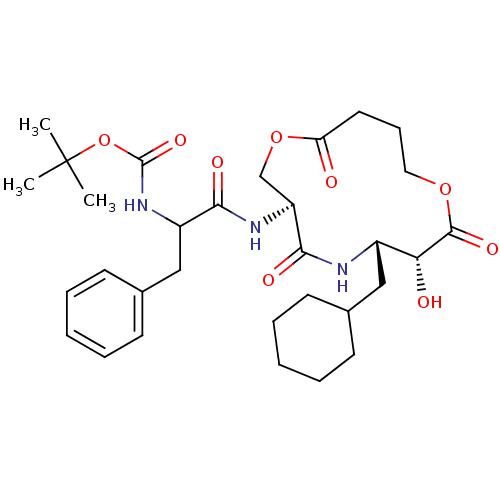 ((diastereomer-1)[1-(6-Cyclohexylmethyl-7-hydroxy-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003189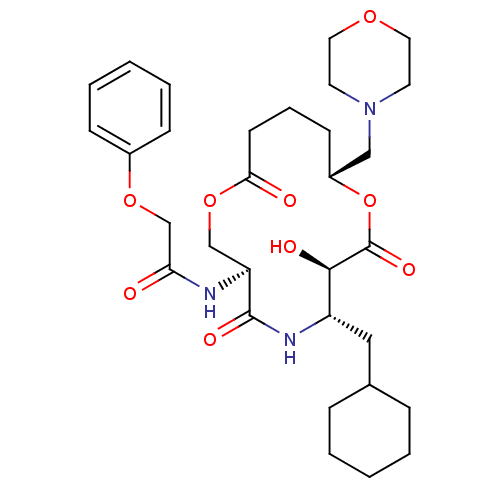 (CHEMBL122919 | N-(6-Cyclohexylmethyl-7-hydroxy-10-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003167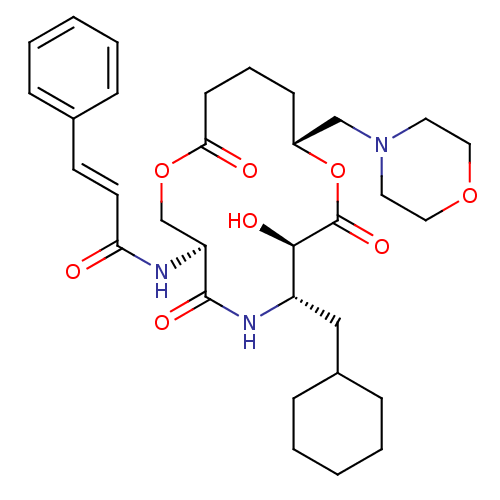 (CHEMBL121731 | N-(6-Cyclohexylmethyl-7-hydroxy-10-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003185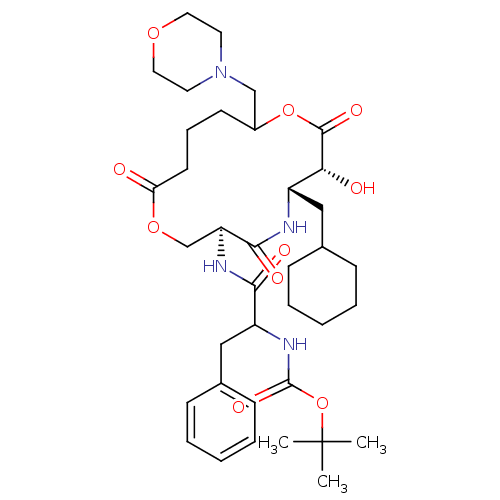 ((diastereomer-1) [1-(6-Cyclohexylmethyl-7-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003185 ((diastereomer-1) [1-(6-Cyclohexylmethyl-7-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003170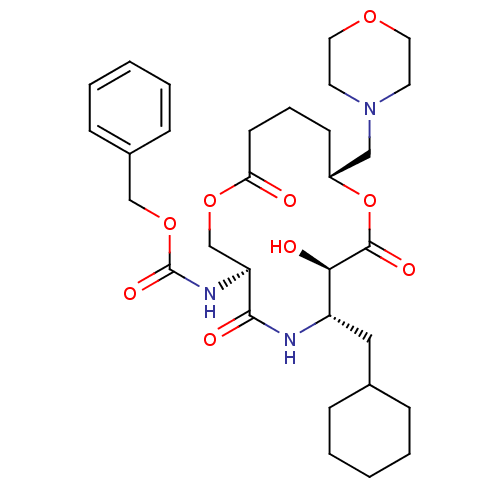 ((6-Cyclohexylmethyl-7-hydroxy-10-morpholin-4-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003193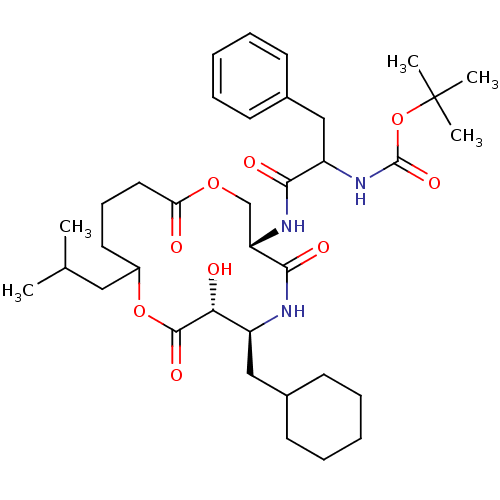 ((diastereomer-1) [1-(6-Cyclohexylmethyl-7-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003193 ((diastereomer-1) [1-(6-Cyclohexylmethyl-7-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003180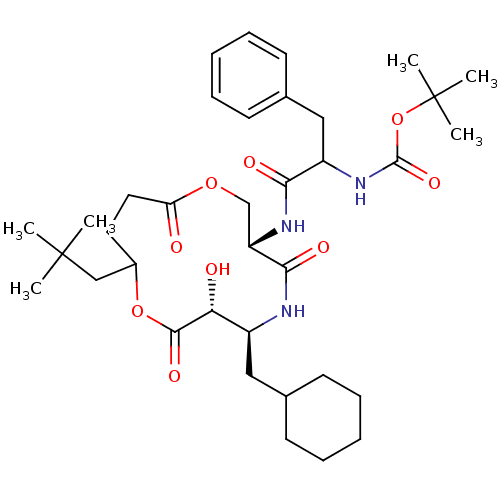 ((diastereomer-1) {1-[6-Cyclohexylmethyl-10-(2,2-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003187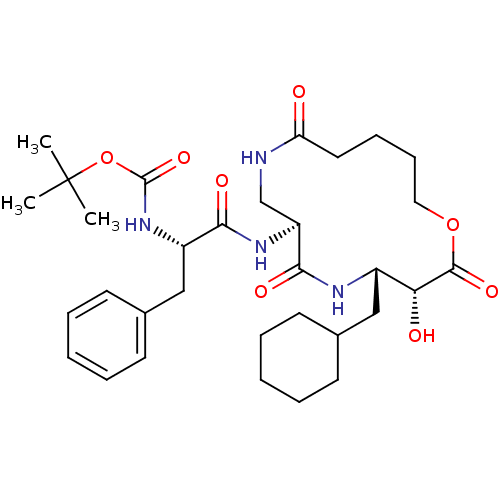 (CHEMBL92538 | [1-(4-Cyclohexylmethyl-3-hydroxy-2,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003178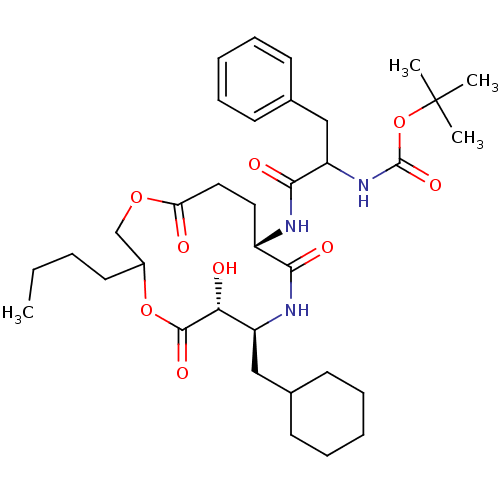 ((diastereomer-1)[1-(3-Butyl-7-cyclohexylmethyl-6-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003178 ((diastereomer-1)[1-(3-Butyl-7-cyclohexylmethyl-6-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003186 (CHEMBL327710 | [1-(4-Cyclohexylmethyl-3-hydroxy-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003179 ((diastereomer-1)[1-(7-Cyclohexylmethyl-6-hydroxy-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003172 ((diastereomer-1) {1-[6-Cyclohexylmethyl-10-(2,2-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003172 ((diastereomer-1) {1-[6-Cyclohexylmethyl-10-(2,2-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003182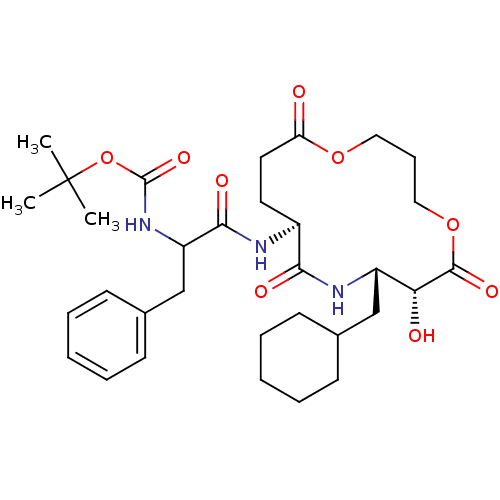 ((diastereomer-1)[1-(8-Cyclohexylmethyl-7-hydroxy-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003184 ((diastereomer-1) [1-(6-Cyclohexylmethyl-7-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003184 ((diastereomer-1) [1-(6-Cyclohexylmethyl-7-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003180 ((diastereomer-1) {1-[6-Cyclohexylmethyl-10-(2,2-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | J Med Chem 35: 3755-73 (1992) BindingDB Entry DOI: 10.7270/Q26W9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||