 Found 55 hits of Enzyme Inhibition Constant Data
Found 55 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230088

(CHEMBL125786)Show SMILES CCCCc1ncc(\C=C(/Cc2ccc(O)c(O)c2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C24H25ClN2O4/c1-2-3-8-23-26-14-19(27(23)15-17-6-4-5-7-20(17)25)13-18(24(30)31)11-16-9-10-21(28)22(29)12-16/h4-7,9-10,12-14,28-29H,2-3,8,11,15H2,1H3,(H,30,31)/b18-13+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003292
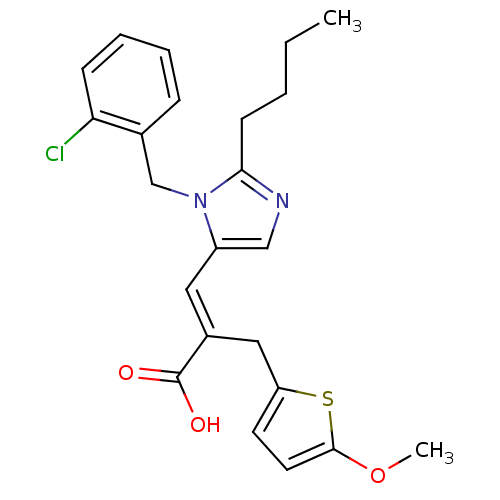
(3-[2-Butyl-3-(2-chloro-benzyl)-3H-imidazol-4-yl]-2...)Show SMILES CCCCc1ncc(\C=C(/Cc2ccc(OC)s2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C23H25ClN2O3S/c1-3-4-9-21-25-14-18(26(21)15-16-7-5-6-8-20(16)24)12-17(23(27)28)13-19-10-11-22(29-2)30-19/h5-8,10-12,14H,3-4,9,13,15H2,1-2H3,(H,27,28)/b17-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-Angiotensin II receptor specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230079
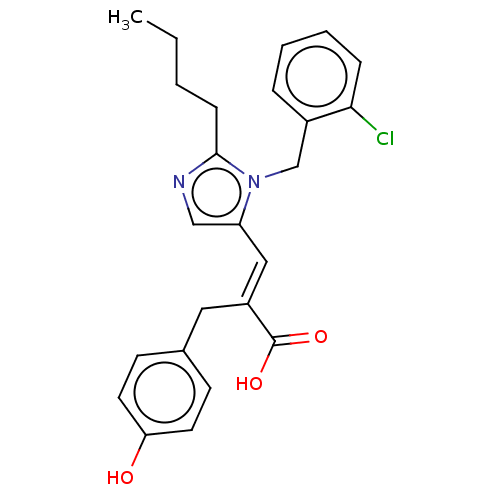
(CHEMBL126594)Show SMILES CCCCc1ncc(\C=C(/Cc2ccc(O)cc2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C24H25ClN2O3/c1-2-3-8-23-26-15-20(27(23)16-18-6-4-5-7-22(18)25)14-19(24(29)30)13-17-9-11-21(28)12-10-17/h4-7,9-12,14-15,28H,2-3,8,13,16H2,1H3,(H,29,30)/b19-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230114
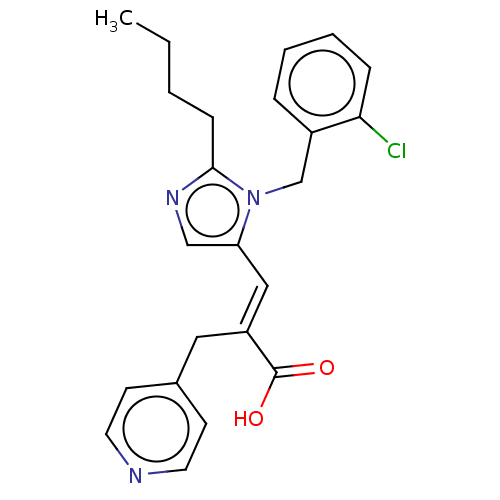
(CHEMBL339961)Show SMILES CCCCc1ncc(\C=C(/Cc2ccncc2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C23H24ClN3O2/c1-2-3-8-22-26-15-20(27(22)16-18-6-4-5-7-21(18)24)14-19(23(28)29)13-17-9-11-25-12-10-17/h4-7,9-12,14-15H,2-3,8,13,16H2,1H3,(H,28,29)/b19-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230103
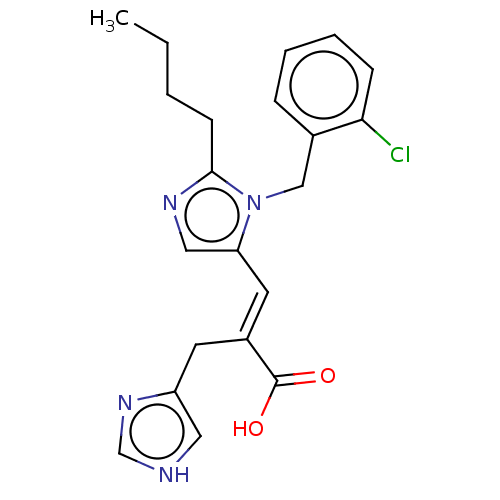
(CHEMBL126356)Show SMILES CCCCc1ncc(\C=C(/Cc2c[nH]cn2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C21H23ClN4O2/c1-2-3-8-20-24-12-18(26(20)13-15-6-4-5-7-19(15)22)10-16(21(27)28)9-17-11-23-14-25-17/h4-7,10-12,14H,2-3,8-9,13H2,1H3,(H,23,25)(H,27,28)/b16-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003285

(3-[2-Butyl-3-(2-chloro-benzyl)-3H-imidazol-4-yl]-2...)Show SMILES CCCCc1ncc(\C=C(/Cc2cc(OC)cs2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C23H25ClN2O3S/c1-3-4-9-22-25-13-18(26(22)14-16-7-5-6-8-21(16)24)10-17(23(27)28)11-20-12-19(29-2)15-30-20/h5-8,10,12-13,15H,3-4,9,11,14H2,1-2H3,(H,27,28)/b17-10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-Angiotensin II receptor specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230105
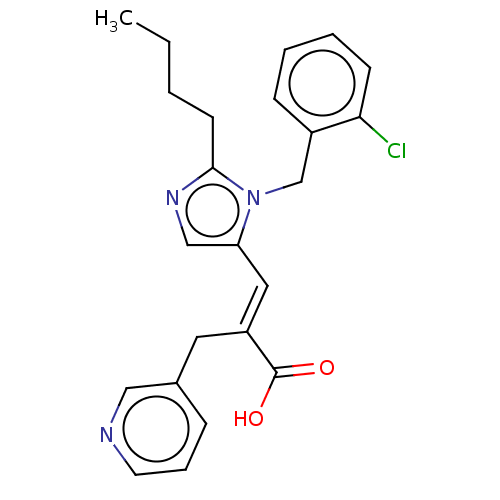
(CHEMBL339446)Show SMILES CCCCc1ncc(\C=C(/Cc2cccnc2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C23H24ClN3O2/c1-2-3-10-22-26-15-20(27(22)16-18-8-4-5-9-21(18)24)13-19(23(28)29)12-17-7-6-11-25-14-17/h4-9,11,13-15H,2-3,10,12,16H2,1H3,(H,28,29)/b19-13+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003265
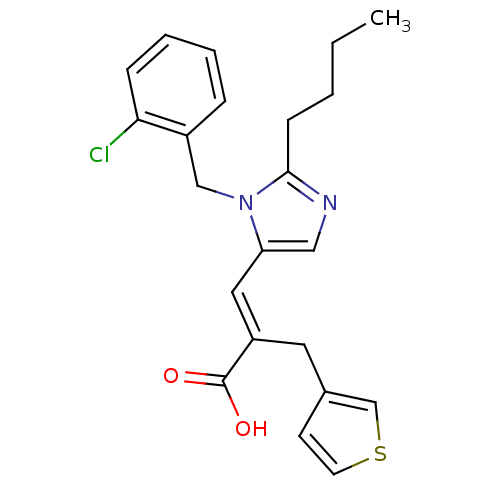
(3-[2-Butyl-3-(2-chloro-benzyl)-3H-imidazol-4-yl]-2...)Show SMILES CCCCc1ncc(\C=C(/Cc2ccsc2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C22H23ClN2O2S/c1-2-3-8-21-24-13-19(25(21)14-17-6-4-5-7-20(17)23)12-18(22(26)27)11-16-9-10-28-15-16/h4-7,9-10,12-13,15H,2-3,8,11,14H2,1H3,(H,26,27)/b18-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-Angiotensin II receptor specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230098
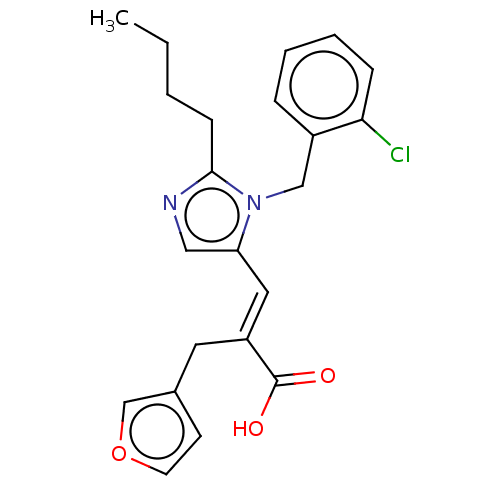
(CHEMBL127005)Show SMILES CCCCc1ncc(\C=C(/Cc2ccoc2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C22H23ClN2O3/c1-2-3-8-21-24-13-19(25(21)14-17-6-4-5-7-20(17)23)12-18(22(26)27)11-16-9-10-28-15-16/h4-7,9-10,12-13,15H,2-3,8,11,14H2,1H3,(H,26,27)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003264
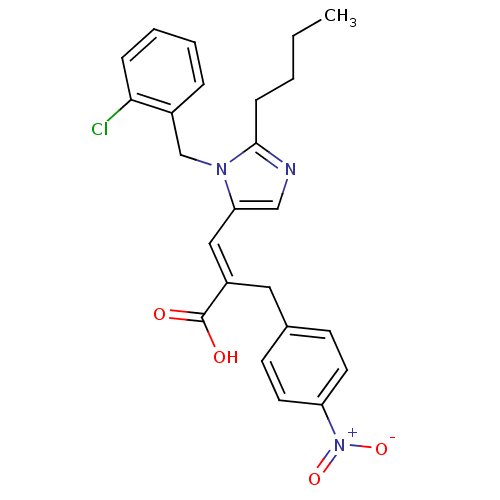
(3-[2-Butyl-3-(2-chloro-benzyl)-3H-imidazol-4-yl]-2...)Show SMILES CCCCc1ncc(\C=C(/Cc2ccc(cc2)[N+]([O-])=O)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C24H24ClN3O4/c1-2-3-8-23-26-15-21(27(23)16-18-6-4-5-7-22(18)25)14-19(24(29)30)13-17-9-11-20(12-10-17)28(31)32/h4-7,9-12,14-15H,2-3,8,13,16H2,1H3,(H,29,30)/b19-14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-Angiotensin II receptor specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003266
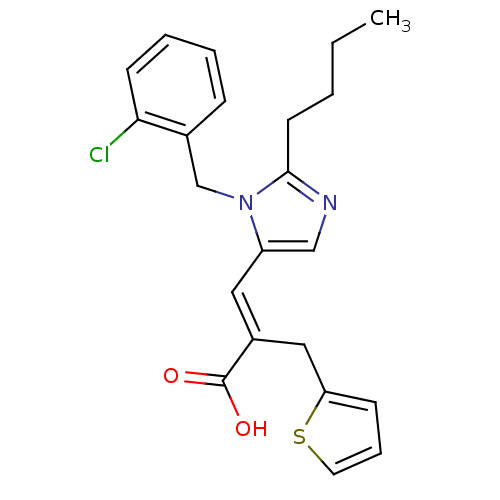
((E)-3-[2-Butyl-3-(2-chloro-benzyl)-3H-imidazol-4-y...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C22H23ClN2O2S/c1-2-3-10-21-24-14-18(25(21)15-16-7-4-5-9-20(16)23)12-17(22(26)27)13-19-8-6-11-28-19/h4-9,11-12,14H,2-3,10,13,15H2,1H3,(H,26,27)/b17-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-Angiotensin II receptor specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230115

(CHEMBL338478)Show SMILES CCCCc1ncc(\C=C(/Cc2ccc3OCOc3c2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C25H25ClN2O4/c1-2-3-8-24-27-14-20(28(24)15-18-6-4-5-7-21(18)26)13-19(25(29)30)11-17-9-10-22-23(12-17)32-16-31-22/h4-7,9-10,12-14H,2-3,8,11,15-16H2,1H3,(H,29,30)/b19-13+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003288
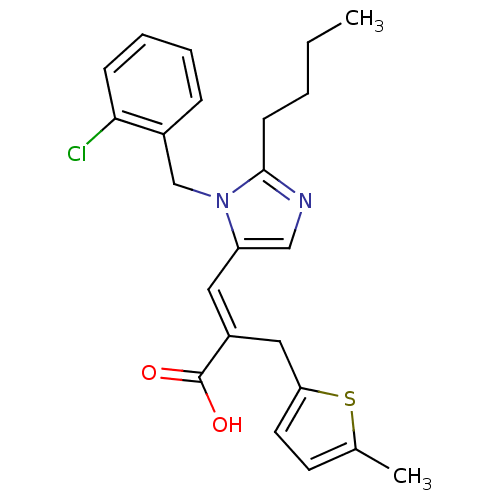
(3-[2-Butyl-3-(2-chloro-benzyl)-3H-imidazol-4-yl]-2...)Show SMILES CCCCc1ncc(\C=C(/Cc2ccc(C)s2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C23H25ClN2O2S/c1-3-4-9-22-25-14-19(26(22)15-17-7-5-6-8-21(17)24)12-18(23(27)28)13-20-11-10-16(2)29-20/h5-8,10-12,14H,3-4,9,13,15H2,1-2H3,(H,27,28)/b18-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-Angiotensin II receptor specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003271
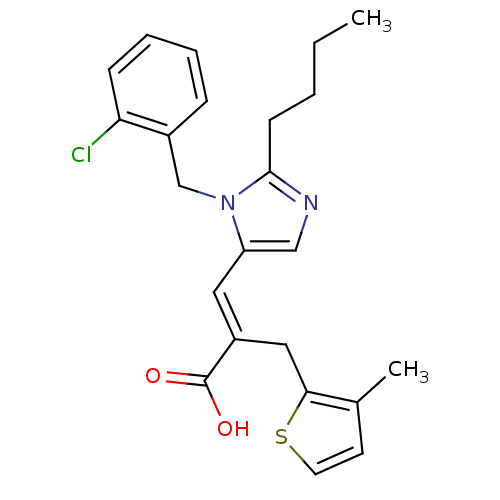
(3-[2-Butyl-3-(2-chloro-benzyl)-3H-imidazol-4-yl]-2...)Show SMILES CCCCc1ncc(\C=C(/Cc2sccc2C)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C23H25ClN2O2S/c1-3-4-9-22-25-14-19(26(22)15-17-7-5-6-8-20(17)24)12-18(23(27)28)13-21-16(2)10-11-29-21/h5-8,10-12,14H,3-4,9,13,15H2,1-2H3,(H,27,28)/b18-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-Angiotensin II receptor specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230096
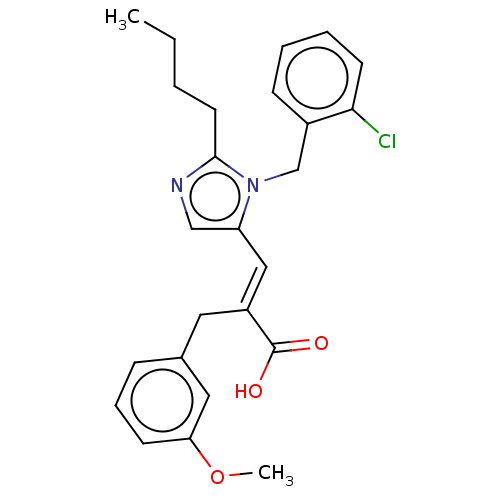
(CHEMBL338517)Show SMILES CCCCc1ncc(\C=C(/Cc2cccc(OC)c2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C25H27ClN2O3/c1-3-4-12-24-27-16-21(28(24)17-19-9-5-6-11-23(19)26)15-20(25(29)30)13-18-8-7-10-22(14-18)31-2/h5-11,14-16H,3-4,12-13,17H2,1-2H3,(H,29,30)/b20-15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230085
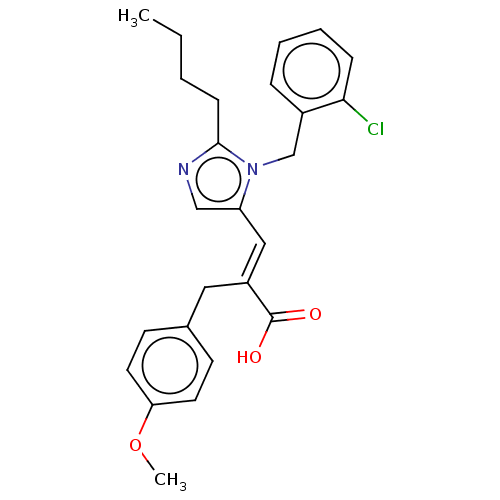
(CHEMBL126359)Show SMILES CCCCc1ncc(\C=C(/Cc2ccc(OC)cc2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C25H27ClN2O3/c1-3-4-9-24-27-16-21(28(24)17-19-7-5-6-8-23(19)26)15-20(25(29)30)14-18-10-12-22(31-2)13-11-18/h5-8,10-13,15-16H,3-4,9,14,17H2,1-2H3,(H,29,30)/b20-15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230080
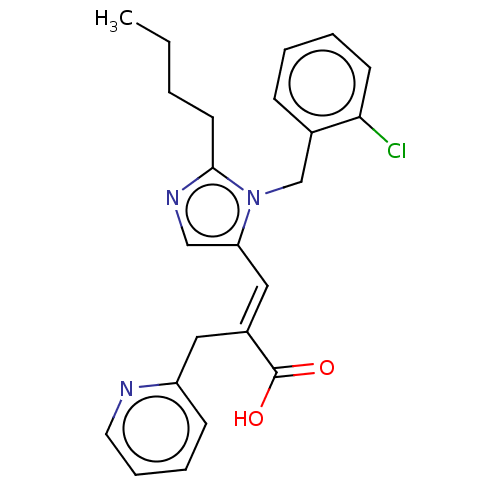
(CHEMBL339860)Show SMILES CCCCc1ncc(\C=C(/Cc2ccccn2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C23H24ClN3O2/c1-2-3-11-22-26-15-20(27(22)16-17-8-4-5-10-21(17)24)14-18(23(28)29)13-19-9-6-7-12-25-19/h4-10,12,14-15H,2-3,11,13,16H2,1H3,(H,28,29)/b18-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230109
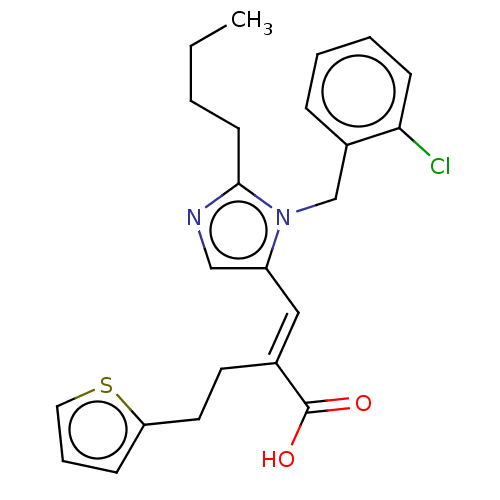
(CHEMBL127379)Show SMILES CCCCc1ncc(\C=C(/CCc2cccs2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C23H25ClN2O2S/c1-2-3-10-22-25-15-19(26(22)16-18-7-4-5-9-21(18)24)14-17(23(27)28)11-12-20-8-6-13-29-20/h4-9,13-15H,2-3,10-12,16H2,1H3,(H,27,28)/b17-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230089

(CHEMBL436594)Show SMILES CCCCc1ncc(\C=C(/Cc2ccco2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C22H23ClN2O3/c1-2-3-10-21-24-14-18(25(21)15-16-7-4-5-9-20(16)23)12-17(22(26)27)13-19-8-6-11-28-19/h4-9,11-12,14H,2-3,10,13,15H2,1H3,(H,26,27)/b17-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230083
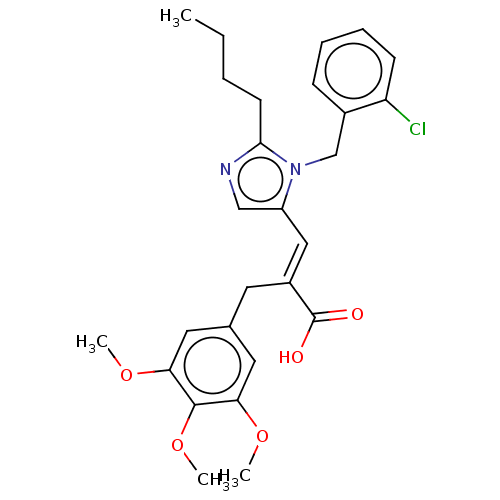
(CHEMBL444069)Show SMILES CCCCc1ncc(\C=C(/Cc2cc(OC)c(OC)c(OC)c2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C27H31ClN2O5/c1-5-6-11-25-29-16-21(30(25)17-19-9-7-8-10-22(19)28)15-20(27(31)32)12-18-13-23(33-2)26(35-4)24(14-18)34-3/h7-10,13-16H,5-6,11-12,17H2,1-4H3,(H,31,32)/b20-15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003269

(3-[2-Butyl-3-(2-chloro-benzyl)-3H-imidazol-4-yl]-2...)Show SMILES CCCCc1ncc(\C=C(/C(c2cccs2)c2ccccc2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C28H27ClN2O2S/c1-2-3-15-26-30-18-22(31(26)19-21-12-7-8-13-24(21)29)17-23(28(32)33)27(25-14-9-16-34-25)20-10-5-4-6-11-20/h4-14,16-18,27H,2-3,15,19H2,1H3,(H,32,33)/b23-17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-Angiotensin II receptor specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003301
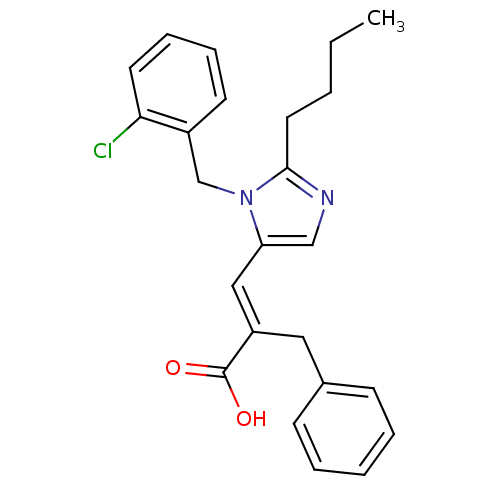
((Z-isomer)-2-Benzyl-3-[2-butyl-3-(2-chloro-benzyl)...)Show SMILES CCCCc1ncc(\C=C(/Cc2ccccc2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C24H25ClN2O2/c1-2-3-13-23-26-16-21(27(23)17-19-11-7-8-12-22(19)25)15-20(24(28)29)14-18-9-5-4-6-10-18/h4-12,15-16H,2-3,13-14,17H2,1H3,(H,28,29)/b20-15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-Angiotensin II receptor specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230094
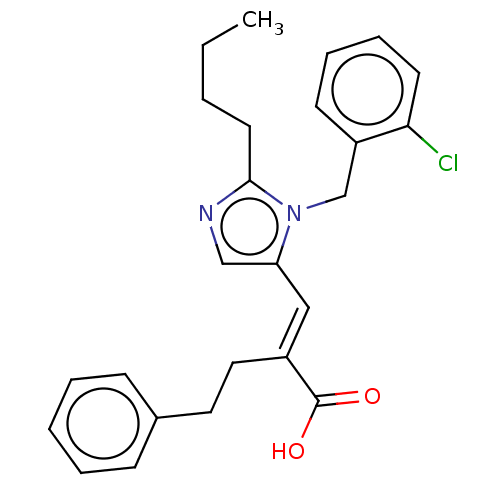
(CHEMBL127308)Show SMILES CCCCc1ncc(\C=C(/CCc2ccccc2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C25H27ClN2O2/c1-2-3-13-24-27-17-22(28(24)18-21-11-7-8-12-23(21)26)16-20(25(29)30)15-14-19-9-5-4-6-10-19/h4-12,16-17H,2-3,13-15,18H2,1H3,(H,29,30)/b20-16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003282

(2-(4-Amino-benzyl)-3-[2-butyl-3-(2-chloro-benzyl)-...)Show SMILES CCCCc1ncc(\C=C(/Cc2ccc(N)cc2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C24H26ClN3O2/c1-2-3-8-23-27-15-21(28(23)16-18-6-4-5-7-22(18)25)14-19(24(29)30)13-17-9-11-20(26)12-10-17/h4-7,9-12,14-15H,2-3,8,13,16,26H2,1H3,(H,29,30)/b19-14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-Angiotensin II receptor specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230111
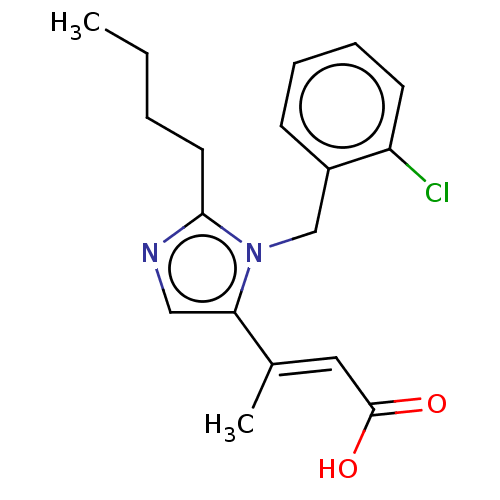
(CHEMBL126501)Show InChI InChI=1S/C18H21ClN2O2/c1-3-4-9-17-20-11-16(13(2)10-18(22)23)21(17)12-14-7-5-6-8-15(14)19/h5-8,10-11H,3-4,9,12H2,1-2H3,(H,22,23)/b13-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230087
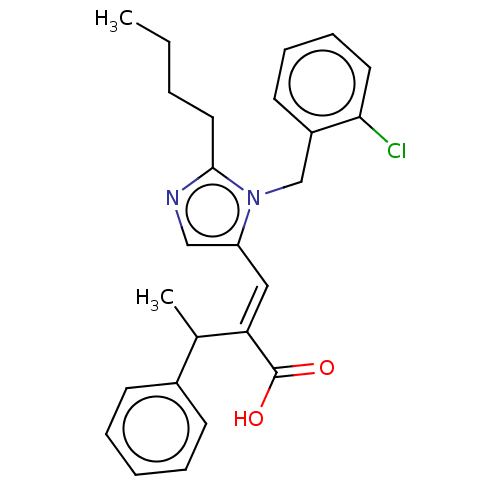
(CHEMBL339623)Show SMILES CCCCc1ncc(\C=C(/C(C)c2ccccc2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C25H27ClN2O2/c1-3-4-14-24-27-16-21(28(24)17-20-12-8-9-13-23(20)26)15-22(25(29)30)18(2)19-10-6-5-7-11-19/h5-13,15-16,18H,3-4,14,17H2,1-2H3,(H,29,30)/b22-15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003279
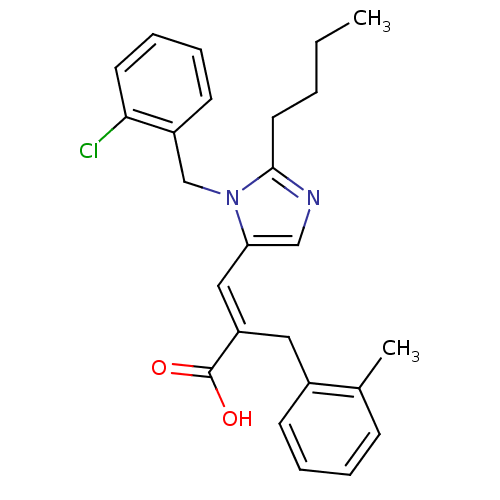
(3-[2-Butyl-3-(2-chloro-benzyl)-3H-imidazol-4-yl]-2...)Show SMILES CCCCc1ncc(\C=C(/Cc2ccccc2C)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C25H27ClN2O2/c1-3-4-13-24-27-16-22(28(24)17-20-11-7-8-12-23(20)26)15-21(25(29)30)14-19-10-6-5-9-18(19)2/h5-12,15-16H,3-4,13-14,17H2,1-2H3,(H,29,30)/b21-15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-Angiotensin II receptor specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230107
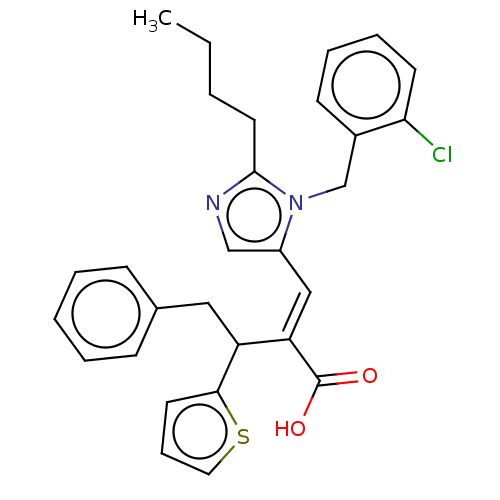
(CHEMBL338067)Show SMILES CCCCc1ncc(\C=C(/C(Cc2ccccc2)c2cccs2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C29H29ClN2O2S/c1-2-3-15-28-31-19-23(32(28)20-22-12-7-8-13-26(22)30)18-25(29(33)34)24(27-14-9-16-35-27)17-21-10-5-4-6-11-21/h4-14,16,18-19,24H,2-3,15,17,20H2,1H3,(H,33,34)/b25-18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230116
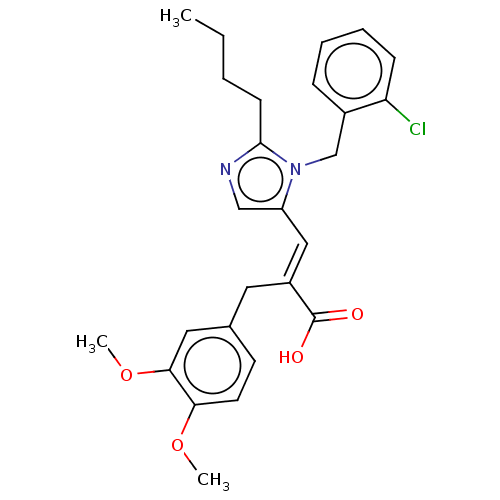
(CHEMBL338249)Show SMILES CCCCc1ncc(\C=C(/Cc2ccc(OC)c(OC)c2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C26H29ClN2O4/c1-4-5-10-25-28-16-21(29(25)17-19-8-6-7-9-22(19)27)15-20(26(30)31)13-18-11-12-23(32-2)24(14-18)33-3/h6-9,11-12,14-16H,4-5,10,13,17H2,1-3H3,(H,30,31)/b20-15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230095
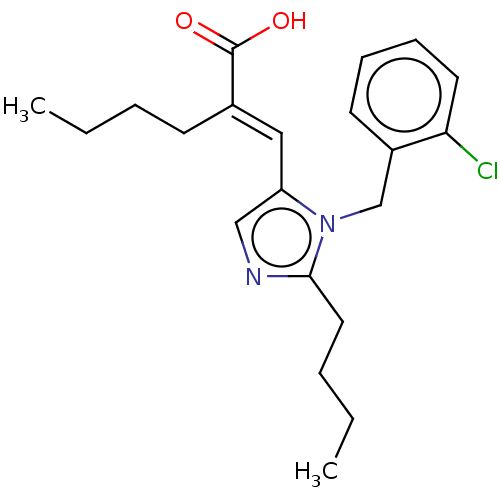
(CHEMBL405525)Show InChI InChI=1S/C21H27ClN2O2/c1-3-5-9-16(21(25)26)13-18-14-23-20(12-6-4-2)24(18)15-17-10-7-8-11-19(17)22/h7-8,10-11,13-14H,3-6,9,12,15H2,1-2H3,(H,25,26)/b16-13+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230108

(CHEMBL126500)Show InChI InChI=1S/C17H19ClN2O2/c1-2-3-8-16-19-11-14(9-10-17(21)22)20(16)12-13-6-4-5-7-15(13)18/h4-7,9-11H,2-3,8,12H2,1H3,(H,21,22)/b10-9- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230112
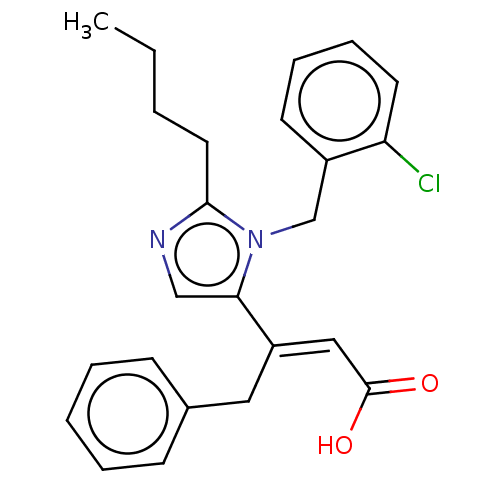
(CHEMBL338690)Show SMILES CCCCc1ncc(\C(Cc2ccccc2)=C\C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C24H25ClN2O2/c1-2-3-13-23-26-16-22(27(23)17-19-11-7-8-12-21(19)25)20(15-24(28)29)14-18-9-5-4-6-10-18/h4-12,15-16H,2-3,13-14,17H2,1H3,(H,28,29)/b20-15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003254
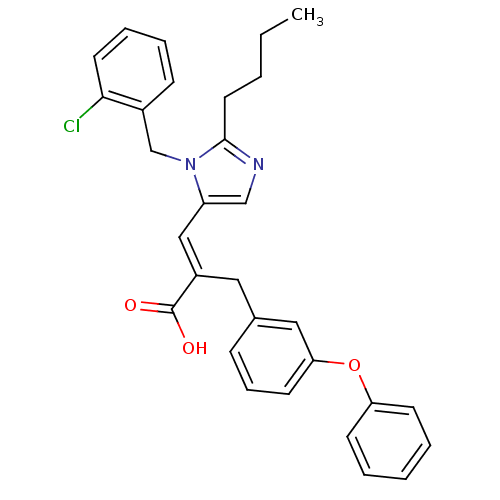
(3-[2-Butyl-3-(2-chloro-benzyl)-3H-imidazol-4-yl]-2...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccc(Oc3ccccc3)c2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C30H29ClN2O3/c1-2-3-16-29-32-20-25(33(29)21-23-11-7-8-15-28(23)31)19-24(30(34)35)17-22-10-9-14-27(18-22)36-26-12-5-4-6-13-26/h4-15,18-20H,2-3,16-17,21H2,1H3,(H,34,35)/b24-19+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of [125I]-AII specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230118
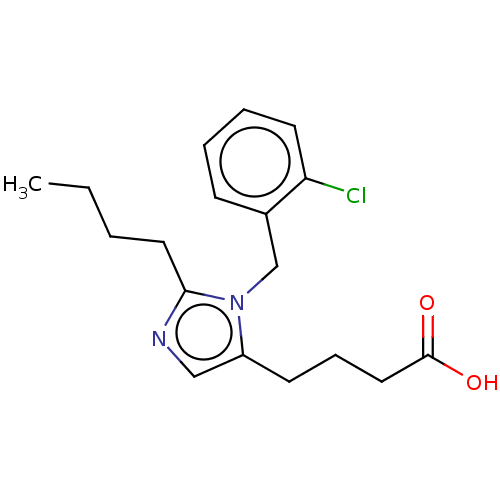
(CHEMBL340917)Show InChI InChI=1S/C18H23ClN2O2/c1-2-3-10-17-20-12-15(8-6-11-18(22)23)21(17)13-14-7-4-5-9-16(14)19/h4-5,7,9,12H,2-3,6,8,10-11,13H2,1H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003290
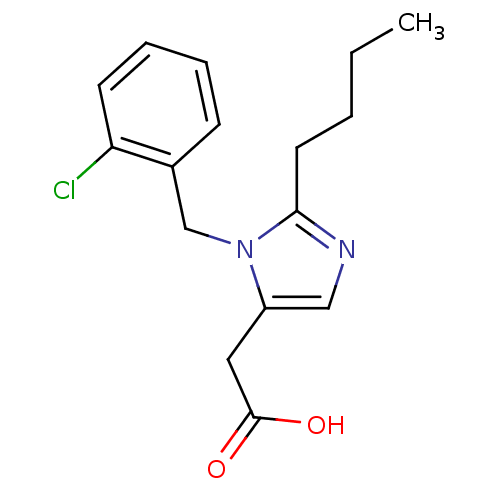
(CHEMBL39345 | [2-Butyl-3-(2-chloro-benzyl)-3H-imid...)Show InChI InChI=1S/C16H19ClN2O2/c1-2-3-8-15-18-10-13(9-16(20)21)19(15)11-12-6-4-5-7-14(12)17/h4-7,10H,2-3,8-9,11H2,1H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Angiotensin II receptor induced vasoconstriction of the rabbit aorta |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230090
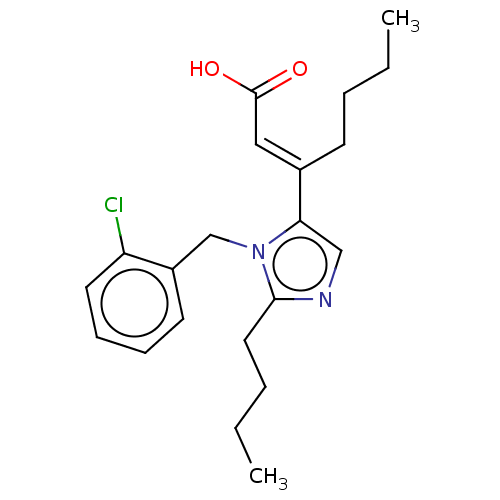
(CHEMBL127916)Show InChI InChI=1S/C21H27ClN2O2/c1-3-5-9-16(13-21(25)26)19-14-23-20(12-6-4-2)24(19)15-17-10-7-8-11-18(17)22/h7-8,10-11,13-14H,3-6,9,12,15H2,1-2H3,(H,25,26)/b16-13+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230086
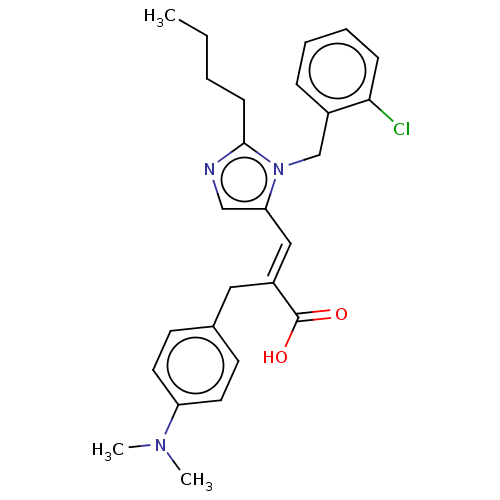
(CHEMBL339939)Show SMILES CCCCc1ncc(\C=C(/Cc2ccc(cc2)N(C)C)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C26H30ClN3O2/c1-4-5-10-25-28-17-23(30(25)18-20-8-6-7-9-24(20)27)16-21(26(31)32)15-19-11-13-22(14-12-19)29(2)3/h6-9,11-14,16-17H,4-5,10,15,18H2,1-3H3,(H,31,32)/b21-16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230104
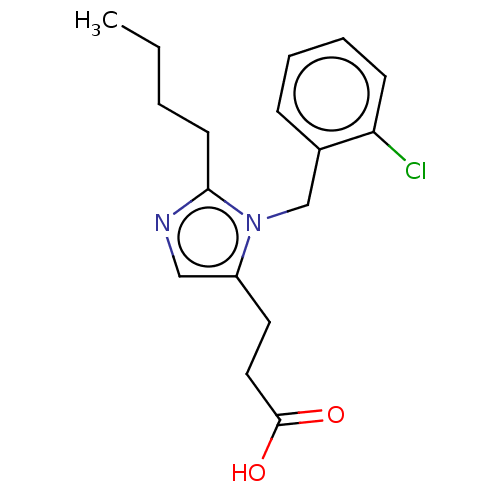
(CHEMBL127515)Show InChI InChI=1S/C17H21ClN2O2/c1-2-3-8-16-19-11-14(9-10-17(21)22)20(16)12-13-6-4-5-7-15(13)18/h4-7,11H,2-3,8-10,12H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230102
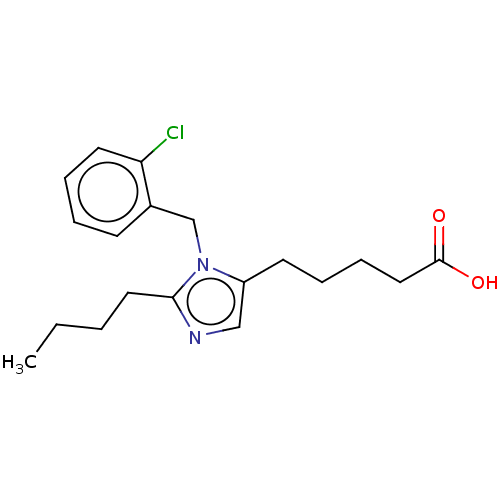
(CHEMBL127566)Show InChI InChI=1S/C19H25ClN2O2/c1-2-3-11-18-21-13-16(9-5-7-12-19(23)24)22(18)14-15-8-4-6-10-17(15)20/h4,6,8,10,13H,2-3,5,7,9,11-12,14H2,1H3,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230106
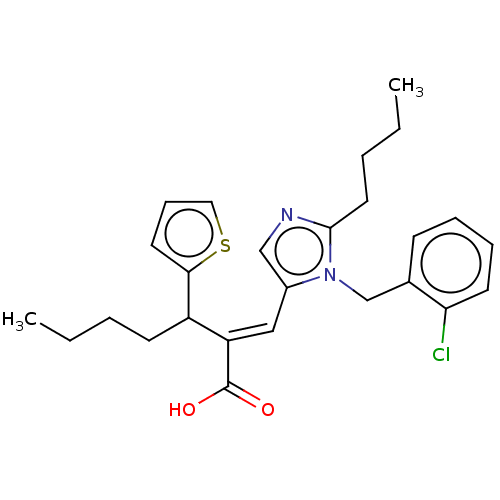
(CHEMBL125341)Show SMILES CCCCC(\C(=C/c1cnc(CCCC)n1Cc1ccccc1Cl)C(O)=O)c1cccs1 Show InChI InChI=1S/C26H31ClN2O2S/c1-3-5-11-21(24-13-9-15-32-24)22(26(30)31)16-20-17-28-25(14-6-4-2)29(20)18-19-10-7-8-12-23(19)27/h7-10,12-13,15-17,21H,3-6,11,14,18H2,1-2H3,(H,30,31)/b22-16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230101
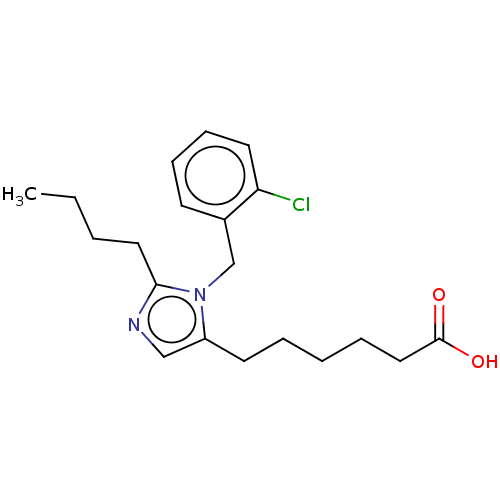
(CHEMBL127137)Show InChI InChI=1S/C20H27ClN2O2/c1-2-3-12-19-22-14-17(10-5-4-6-13-20(24)25)23(19)15-16-9-7-8-11-18(16)21/h7-9,11,14H,2-6,10,12-13,15H2,1H3,(H,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230097
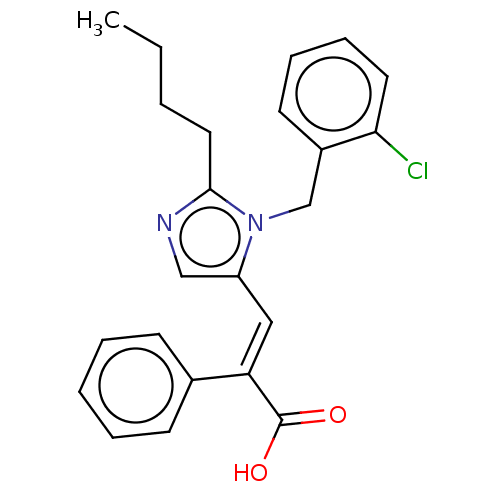
(CHEMBL434254)Show SMILES CCCCc1ncc(\C=C(\C(O)=O)c2ccccc2)n1Cc1ccccc1Cl Show InChI InChI=1S/C23H23ClN2O2/c1-2-3-13-22-25-15-19(26(22)16-18-11-7-8-12-21(18)24)14-20(23(27)28)17-9-5-4-6-10-17/h4-12,14-15H,2-3,13,16H2,1H3,(H,27,28)/b20-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230113
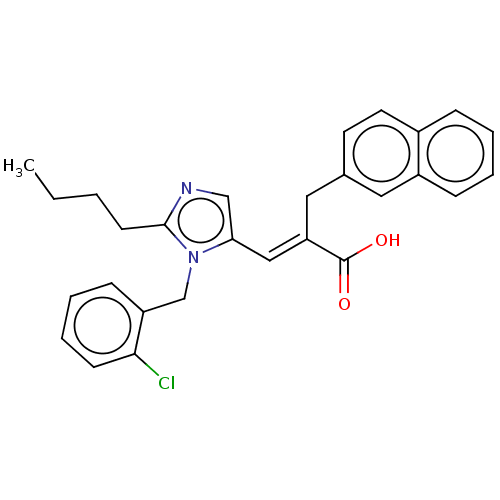
(CHEMBL435804)Show SMILES CCCCc1ncc(\C=C(/Cc2ccc3ccccc3c2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C28H27ClN2O2/c1-2-3-12-27-30-18-25(31(27)19-23-10-6-7-11-26(23)29)17-24(28(32)33)16-20-13-14-21-8-4-5-9-22(21)15-20/h4-11,13-15,17-18H,2-3,12,16,19H2,1H3,(H,32,33)/b24-17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230099
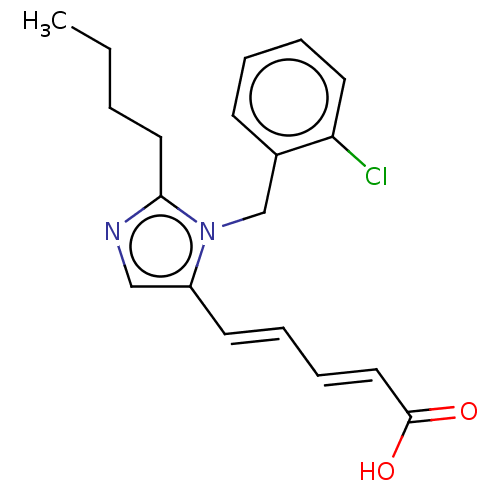
(CHEMBL340433)Show InChI InChI=1S/C19H21ClN2O2/c1-2-3-11-18-21-13-16(9-5-7-12-19(23)24)22(18)14-15-8-4-6-10-17(15)20/h4-10,12-13H,2-3,11,14H2,1H3,(H,23,24)/b9-5+,12-7+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230084

(CHEMBL338423)Show InChI InChI=1S/C18H21ClN2O2/c1-3-4-9-17-20-11-15(10-13(2)18(22)23)21(17)12-14-7-5-6-8-16(14)19/h5-8,10-11H,3-4,9,12H2,1-2H3,(H,22,23)/b13-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230117
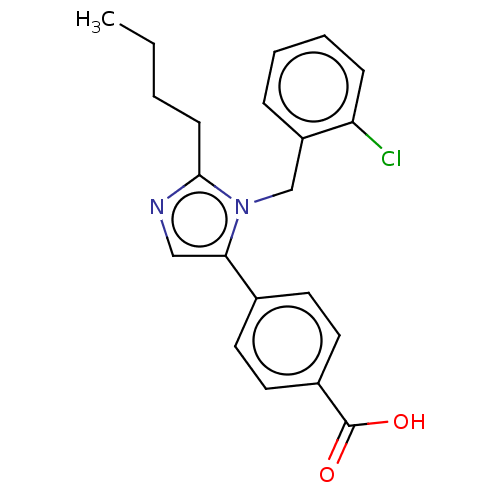
(CHEMBL126357)Show InChI InChI=1S/C21H21ClN2O2/c1-2-3-8-20-23-13-19(15-9-11-16(12-10-15)21(25)26)24(20)14-17-6-4-5-7-18(17)22/h4-7,9-13H,2-3,8,14H2,1H3,(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003278
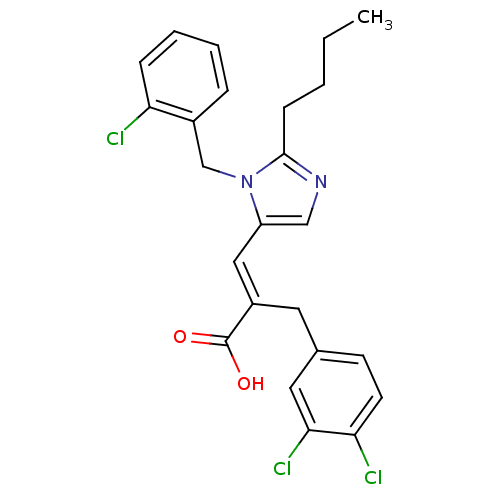
(3-[2-Butyl-3-(2-chloro-benzyl)-3H-imidazol-4-yl]-2...)Show SMILES CCCCc1ncc(\C=C(/Cc2ccc(Cl)c(Cl)c2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C24H23Cl3N2O2/c1-2-3-8-23-28-14-19(29(23)15-17-6-4-5-7-20(17)25)13-18(24(30)31)11-16-9-10-21(26)22(27)12-16/h4-7,9-10,12-14H,2-3,8,11,15H2,1H3,(H,30,31)/b18-13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-Angiotensin II receptor specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230091
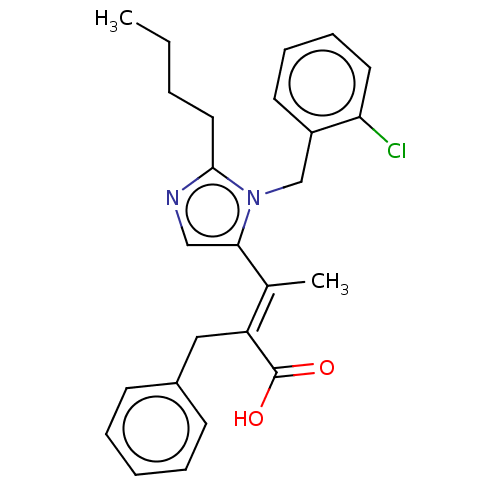
(CHEMBL340316)Show SMILES CCCCc1ncc(\C(C)=C(/Cc2ccccc2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C25H27ClN2O2/c1-3-4-14-24-27-16-23(28(24)17-20-12-8-9-13-22(20)26)18(2)21(25(29)30)15-19-10-6-5-7-11-19/h5-13,16H,3-4,14-15,17H2,1-2H3,(H,29,30)/b21-18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for binding affinity against human carbonic anhydrase II; (ki*10e-9) |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230082
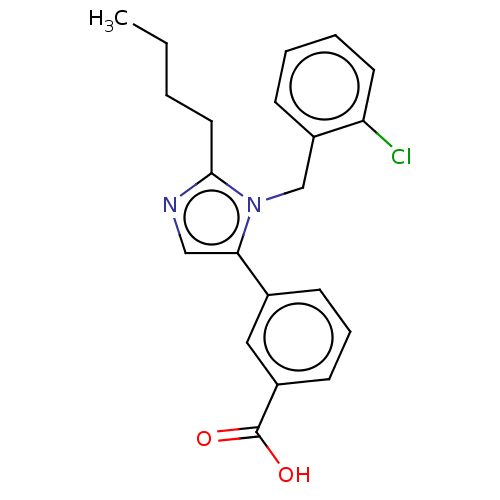
(CHEMBL339121)Show InChI InChI=1S/C21H21ClN2O2/c1-2-3-11-20-23-13-19(15-8-6-9-16(12-15)21(25)26)24(20)14-17-7-4-5-10-18(17)22/h4-10,12-13H,2-3,11,14H2,1H3,(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230110
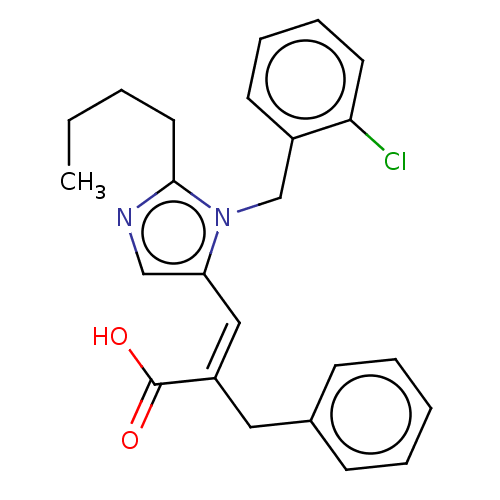
(CHEMBL353234)Show SMILES CCCCc1ncc(\C=C(\Cc2ccccc2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C24H25ClN2O2/c1-2-3-13-23-26-16-21(27(23)17-19-11-7-8-12-22(19)25)15-20(24(28)29)14-18-9-5-4-6-10-18/h4-12,15-16H,2-3,13-14,17H2,1H3,(H,28,29)/b20-15- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50003284
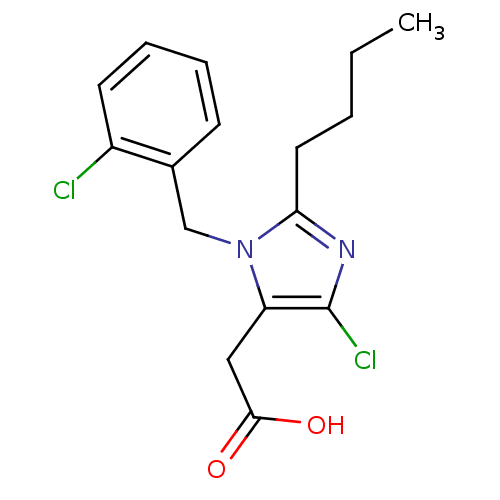
(CHEMBL25528 | [2-Butyl-5-chloro-3-(2-chloro-benzyl...)Show InChI InChI=1S/C16H18Cl2N2O2/c1-2-3-8-14-19-16(18)13(9-15(21)22)20(14)10-11-6-4-5-7-12(11)17/h4-7H,2-3,8-10H2,1H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-Angiotensin II receptor specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230092
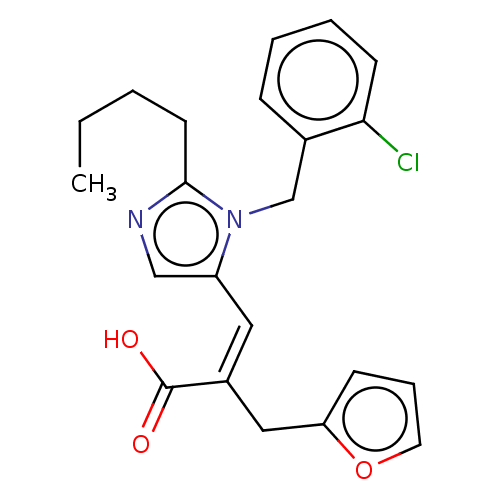
(CHEMBL2111841)Show SMILES CCCCc1ncc(\C=C(\Cc2ccco2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C22H23ClN2O3/c1-2-3-10-21-24-14-18(25(21)15-16-7-4-5-9-20(16)23)12-17(22(26)27)13-19-8-6-11-28-19/h4-9,11-12,14H,2-3,10,13,15H2,1H3,(H,26,27)/b17-12- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230081
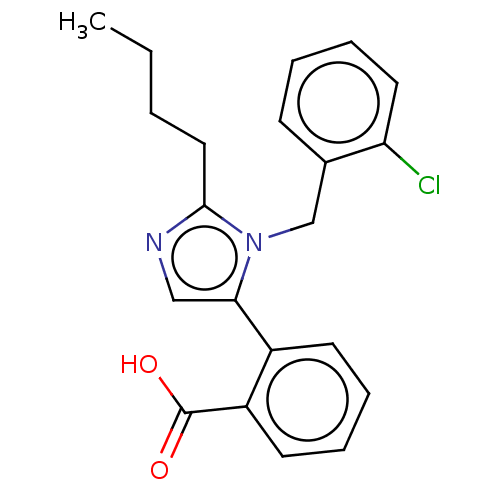
(CHEMBL420515)Show InChI InChI=1S/C21H21ClN2O2/c1-2-3-12-20-23-13-19(16-9-5-6-10-17(16)21(25)26)24(20)14-15-8-4-7-11-18(15)22/h4-11,13H,2-3,12,14H2,1H3,(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50003257

(3-[2-Butyl-3-(2-chloro-benzyl)-3H-imidazol-4-yl]-a...)Show InChI InChI=1S/C17H19ClN2O2/c1-2-3-8-16-19-11-14(9-10-17(21)22)20(16)12-13-6-4-5-7-15(13)18/h4-7,9-11H,2-3,8,12H2,1H3,(H,21,22)/b10-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230093
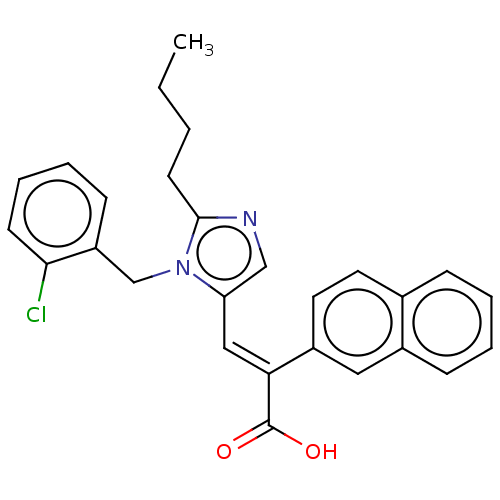
(CHEMBL341011)Show SMILES CCCCc1ncc(\C=C(\C(O)=O)c2ccc3ccccc3c2)n1Cc1ccccc1Cl Show InChI InChI=1S/C27H25ClN2O2/c1-2-3-12-26-29-17-23(30(26)18-22-10-6-7-11-25(22)28)16-24(27(31)32)21-14-13-19-8-4-5-9-20(19)15-21/h4-11,13-17H,2-3,12,18H2,1H3,(H,31,32)/b24-16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]angiotensin II specific binding to rat mesenteric arteries |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data