

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (RAT) | BDBM50003669 (Ac-Tyr(SO3H)-Met-Gly-Trp-Met-R-Dtc-Phe-NH2 | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003666 (3-[2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity against cholecystokinin type A receptor from rat pancreas binding assay | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003670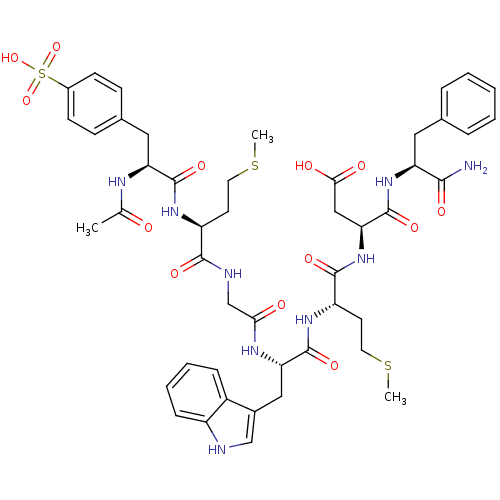 (3-{2-[2-(2-{2-[2-Acetylamino-3-(4-sulfo-phenyl)-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003667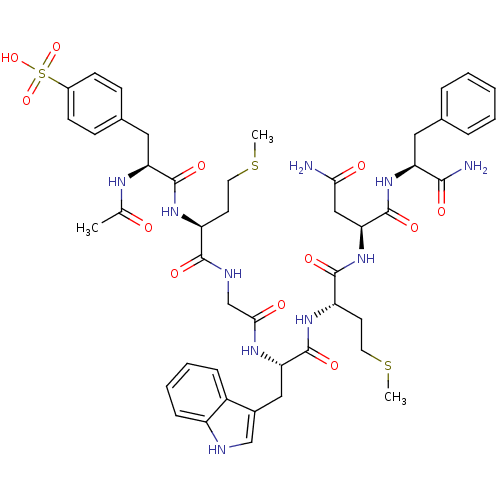 (4-{2-Acetylamino-2-[1-({[1-{1-[2-carbamoyl-1-(1-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003664 (4-{2-Acetylamino-2-[1-({[1-{1-[1-(1-carbamoyl-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003670 (3-{2-[2-(2-{2-[2-Acetylamino-3-(4-sulfo-phenyl)-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration of the compound inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type B receptor of bovine striatum. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003665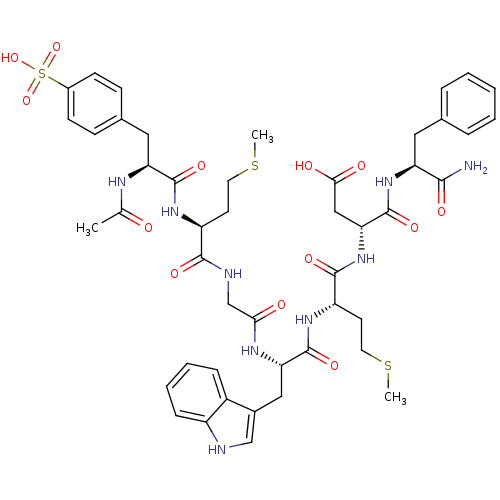 (3-{2-[2-(2-{2-[2-Acetylamino-3-(4-sulfo-phenyl)-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003662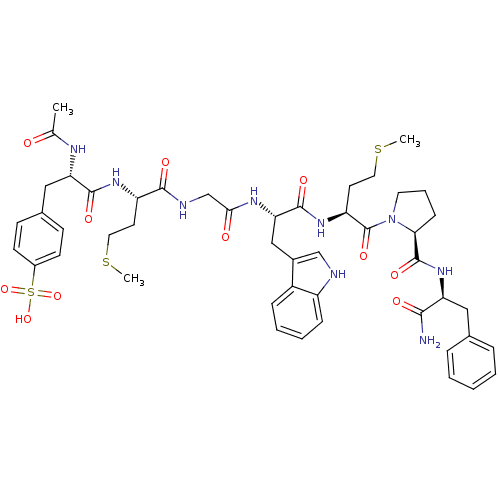 (4-{2-Acetylamino-2-[1-({[1-{1-[2-(1-carbamoyl-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003665 (3-{2-[2-(2-{2-[2-Acetylamino-3-(4-sulfo-phenyl)-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type B receptor of bovine striatum. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003668 (CHEMBL3143146 | [1-[1-[4-(1-Carbamoyl-2-phenyl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity against cholecystokinin type A receptor from rat pancreas binding assay | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003664 (4-{2-Acetylamino-2-[1-({[1-{1-[1-(1-carbamoyl-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type B receptor of bovine striatum. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003667 (4-{2-Acetylamino-2-[1-({[1-{1-[2-carbamoyl-1-(1-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type B receptor of bovine striatum. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50003663 (Ac-Tyr(SO3H)-Met-Gly-Trp-Met-S-Dtc-Phe-NH2 | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 314 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50003666 (3-[2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity against cholecystokinin type A receptor from bovine striatal binding assay | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003669 (Ac-Tyr(SO3H)-Met-Gly-Trp-Met-R-Dtc-Phe-NH2 | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type B receptor of bovine striatum. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003662 (4-{2-Acetylamino-2-[1-({[1-{1-[2-(1-carbamoyl-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type B receptor of bovine striatum. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Bos taurus) | BDBM50003663 (Ac-Tyr(SO3H)-Met-Gly-Trp-Met-S-Dtc-Phe-NH2 | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Concentration inhibiting [3H]-propanoyl-CCK-8 binding to cholecystokinin type B receptor of bovine striatum. | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50003668 (CHEMBL3143146 | [1-[1-[4-(1-Carbamoyl-2-phenyl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity against cholecystokinin type A receptor from bovine striatal binding assay | J Med Chem 35: 4249-52 (1992) BindingDB Entry DOI: 10.7270/Q2Z60N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||