 Found 73 hits of Enzyme Inhibition Constant Data
Found 73 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091800
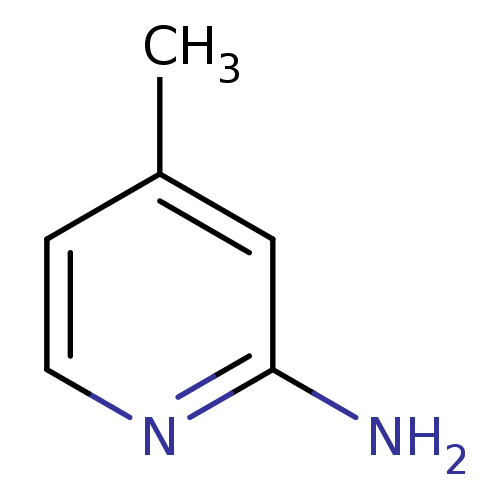
(2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...)Show InChI InChI=1S/C6H8N2/c1-5-2-3-8-6(7)4-5/h2-4H,1H3,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human inducible nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141086

(6-[7-(2-Dimethylamino-ethoxy)-indan-4-yl]-pyridin-...)Show InChI InChI=1S/C18H23N3O/c1-21(2)11-12-22-17-10-9-14(13-5-3-6-15(13)17)16-7-4-8-18(19)20-16/h4,7-10H,3,5-6,11-12H2,1-2H3,(H2,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141082
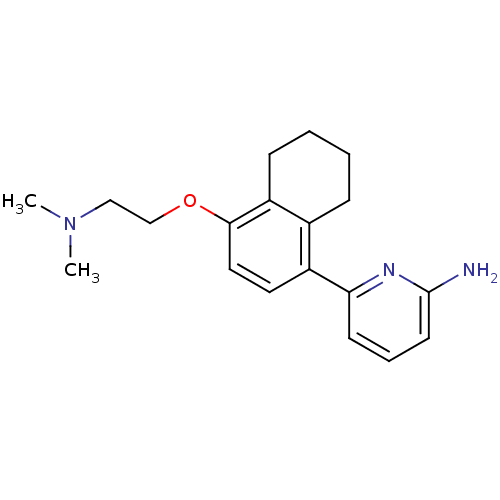
(6-[4-(2-Dimethylamino-ethoxy)-5,6,7,8-tetrahydro-n...)Show InChI InChI=1S/C19H25N3O/c1-22(2)12-13-23-18-11-10-15(14-6-3-4-7-16(14)18)17-8-5-9-19(20)21-17/h5,8-11H,3-4,6-7,12-13H2,1-2H3,(H2,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50091800

(2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...)Show InChI InChI=1S/C6H8N2/c1-5-2-3-8-6(7)4-5/h2-4H,1H3,(H2,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50091800

(2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...)Show InChI InChI=1S/C6H8N2/c1-5-2-3-8-6(7)4-5/h2-4H,1H3,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141081

(6-[4-(1-Methyl-pyrrolidin-3-yloxy)-naphthalen-1-yl...)Show InChI InChI=1S/C20H21N3O/c1-23-12-11-14(13-23)24-19-10-9-16(15-5-2-3-6-17(15)19)18-7-4-8-20(21)22-18/h2-10,14H,11-13H2,1H3,(H2,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50141086

(6-[7-(2-Dimethylamino-ethoxy)-indan-4-yl]-pyridin-...)Show InChI InChI=1S/C18H23N3O/c1-21(2)11-12-22-17-10-9-14(13-5-3-6-15(13)17)16-7-4-8-18(19)20-16/h4,7-10H,3,5-6,11-12H2,1-2H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141089

(6-[7-(2-Pyrrolidin-1-yl-ethoxy)-indan-4-yl]-pyridi...)Show InChI InChI=1S/C20H25N3O/c21-20-8-4-7-18(22-20)16-9-10-19(17-6-3-5-15(16)17)24-14-13-23-11-1-2-12-23/h4,7-10H,1-3,5-6,11-14H2,(H2,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50141081

(6-[4-(1-Methyl-pyrrolidin-3-yloxy)-naphthalen-1-yl...)Show InChI InChI=1S/C20H21N3O/c1-23-12-11-14(13-23)24-19-10-9-16(15-5-2-3-6-17(15)19)18-7-4-8-20(21)22-18/h2-10,14H,11-13H2,1H3,(H2,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50141082

(6-[4-(2-Dimethylamino-ethoxy)-5,6,7,8-tetrahydro-n...)Show InChI InChI=1S/C19H25N3O/c1-22(2)12-13-23-18-11-10-15(14-6-3-4-7-16(14)18)17-8-5-9-19(20)21-17/h5,8-11H,3-4,6-7,12-13H2,1-2H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141085
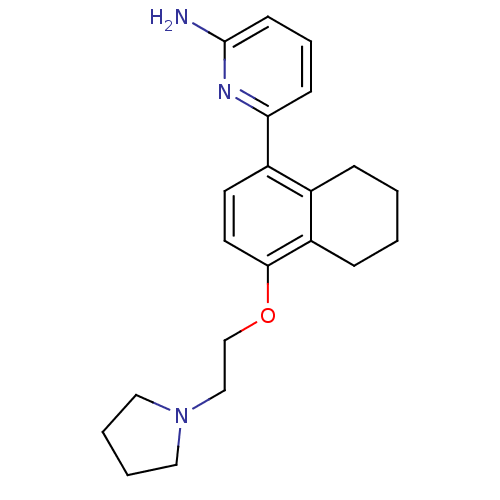
(6-[4-(2-Pyrrolidin-1-yl-ethoxy)-5,6,7,8-tetrahydro...)Show InChI InChI=1S/C21H27N3O/c22-21-9-5-8-19(23-21)17-10-11-20(18-7-2-1-6-16(17)18)25-15-14-24-12-3-4-13-24/h5,8-11H,1-4,6-7,12-15H2,(H2,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141077
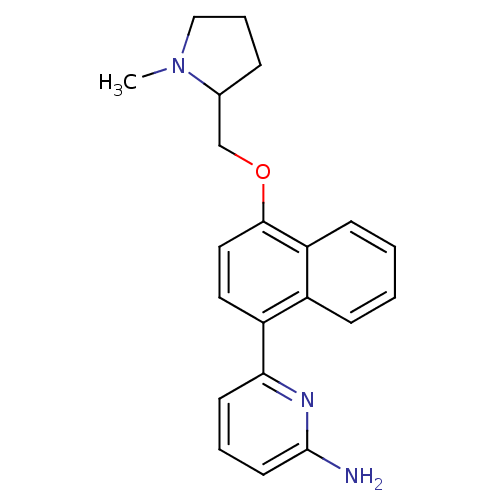
(6-[4-(1-Methyl-pyrrolidin-2-ylmethoxy)-naphthalen-...)Show InChI InChI=1S/C21H23N3O/c1-24-13-5-6-15(24)14-25-20-12-11-17(16-7-2-3-8-18(16)20)19-9-4-10-21(22)23-19/h2-4,7-12,15H,5-6,13-14H2,1H3,(H2,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141074
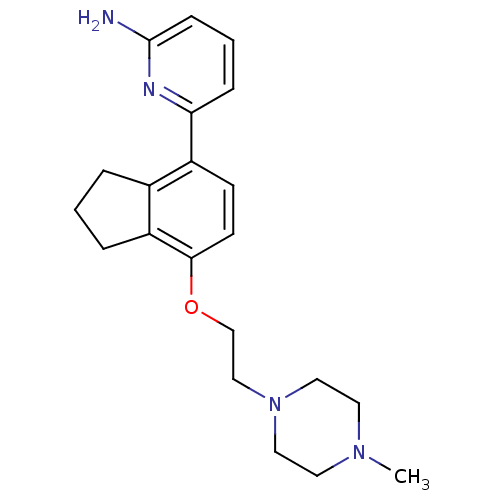
(6-{7-[2-(4-Methyl-piperazin-1-yl)-ethoxy]-indan-4-...)Show InChI InChI=1S/C21H28N4O/c1-24-10-12-25(13-11-24)14-15-26-20-9-8-17(16-4-2-5-18(16)20)19-6-3-7-21(22)23-19/h3,6-9H,2,4-5,10-15H2,1H3,(H2,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141079
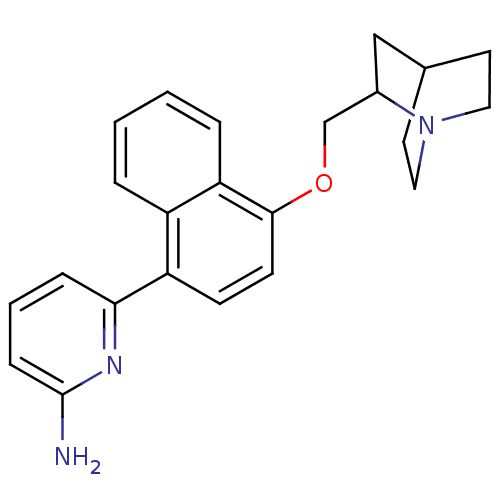
(6-[4-(1-Aza-bicyclo[2.2.2]oct-2-ylmethoxy)-naphtha...)Show SMILES Nc1cccc(n1)-c1ccc(OCC2CC3CCN2CC3)c2ccccc12 |(11.24,-2.54,;9.89,-1.75,;9.89,-.21,;8.56,.56,;7.23,-.21,;7.23,-1.75,;8.56,-2.52,;5.89,-2.52,;4.57,-1.75,;3.24,-2.52,;3.24,-4.06,;1.89,-4.85,;.57,-4.08,;-.76,-4.86,;-2.09,-4.08,;-3.43,-4.85,;-1.9,-4.84,;-2.3,-6.31,;-.76,-6.4,;-2.11,-7.17,;-3.44,-6.4,;4.57,-4.82,;4.57,-6.37,;5.89,-7.14,;7.23,-6.37,;7.23,-4.82,;5.89,-4.06,)| Show InChI InChI=1S/C23H25N3O/c24-23-7-3-6-21(25-23)19-8-9-22(20-5-2-1-4-18(19)20)27-15-17-14-16-10-12-26(17)13-11-16/h1-9,16-17H,10-15H2,(H2,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141088

(6-[4-(2-Dimethylamino-ethoxy)-6,7,8,9-tetrahydro-5...)Show InChI InChI=1S/C20H27N3O/c1-23(2)13-14-24-19-12-11-16(18-9-6-10-20(21)22-18)15-7-4-3-5-8-17(15)19/h6,9-12H,3-5,7-8,13-14H2,1-2H3,(H2,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50141079

(6-[4-(1-Aza-bicyclo[2.2.2]oct-2-ylmethoxy)-naphtha...)Show SMILES Nc1cccc(n1)-c1ccc(OCC2CC3CCN2CC3)c2ccccc12 |(11.24,-2.54,;9.89,-1.75,;9.89,-.21,;8.56,.56,;7.23,-.21,;7.23,-1.75,;8.56,-2.52,;5.89,-2.52,;4.57,-1.75,;3.24,-2.52,;3.24,-4.06,;1.89,-4.85,;.57,-4.08,;-.76,-4.86,;-2.09,-4.08,;-3.43,-4.85,;-1.9,-4.84,;-2.3,-6.31,;-.76,-6.4,;-2.11,-7.17,;-3.44,-6.4,;4.57,-4.82,;4.57,-6.37,;5.89,-7.14,;7.23,-6.37,;7.23,-4.82,;5.89,-4.06,)| Show InChI InChI=1S/C23H25N3O/c24-23-7-3-6-21(25-23)19-8-9-22(20-5-2-1-4-18(19)20)27-15-17-14-16-10-12-26(17)13-11-16/h1-9,16-17H,10-15H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141083
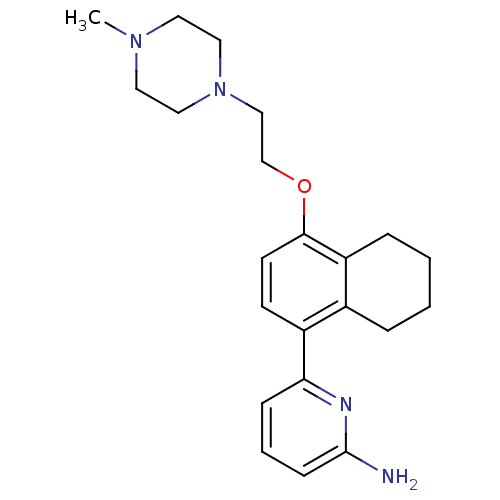
(6-{4-[2-(4-Methyl-piperazin-1-yl)-ethoxy]-5,6,7,8-...)Show InChI InChI=1S/C22H30N4O/c1-25-11-13-26(14-12-25)15-16-27-21-10-9-18(17-5-2-3-6-19(17)21)20-7-4-8-22(23)24-20/h4,7-10H,2-3,5-6,11-16H2,1H3,(H2,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 99.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50091800

(2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...)Show InChI InChI=1S/C6H8N2/c1-5-2-3-8-6(7)4-5/h2-4H,1H3,(H2,7,8) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50141088

(6-[4-(2-Dimethylamino-ethoxy)-6,7,8,9-tetrahydro-5...)Show InChI InChI=1S/C20H27N3O/c1-23(2)13-14-24-19-12-11-16(18-9-6-10-20(21)22-18)15-7-4-3-5-8-17(15)19/h6,9-12H,3-5,7-8,13-14H2,1-2H3,(H2,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50141077

(6-[4-(1-Methyl-pyrrolidin-2-ylmethoxy)-naphthalen-...)Show InChI InChI=1S/C21H23N3O/c1-24-13-5-6-15(24)14-25-20-12-11-17(16-7-2-3-8-18(16)20)19-9-4-10-21(22)23-19/h2-4,7-12,15H,5-6,13-14H2,1H3,(H2,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50141089

(6-[7-(2-Pyrrolidin-1-yl-ethoxy)-indan-4-yl]-pyridi...)Show InChI InChI=1S/C20H25N3O/c21-20-8-4-7-18(22-20)16-9-10-19(17-6-3-5-15(16)17)24-14-13-23-11-1-2-12-23/h4,7-10H,1-3,5-6,11-14H2,(H2,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50141075
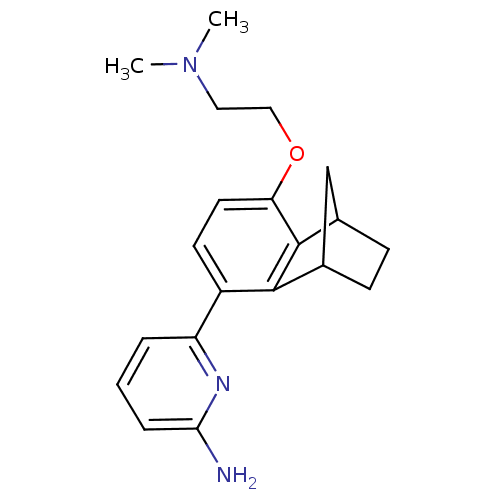
(6-[8-(2-Dimethylamino-ethoxy)-1,2,3,4-tetrahydro-1...)Show InChI InChI=1S/C20H25N3O/c1-23(2)10-11-24-17-9-8-15(16-4-3-5-18(21)22-16)19-13-6-7-14(12-13)20(17)19/h3-5,8-9,13-14H,6-7,10-12H2,1-2H3,(H2,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50141083

(6-{4-[2-(4-Methyl-piperazin-1-yl)-ethoxy]-5,6,7,8-...)Show InChI InChI=1S/C22H30N4O/c1-25-11-13-26(14-12-25)15-16-27-21-10-9-18(17-5-2-3-6-19(17)21)20-7-4-8-22(23)24-20/h4,7-10H,2-3,5-6,11-16H2,1H3,(H2,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50141074

(6-{7-[2-(4-Methyl-piperazin-1-yl)-ethoxy]-indan-4-...)Show InChI InChI=1S/C21H28N4O/c1-24-10-12-25(13-11-24)14-15-26-20-9-8-17(16-4-2-5-18(16)20)19-6-3-7-21(22)23-19/h3,6-9H,2,4-5,10-15H2,1H3,(H2,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50081082
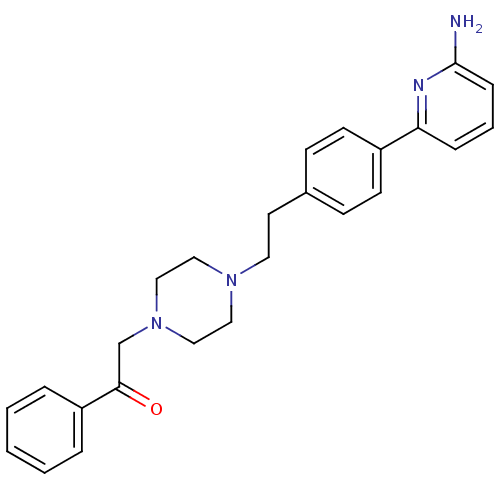
(2-(4-{2-[4-(6-Amino-pyridin-2-yl)-phenyl]-ethyl}-p...)Show SMILES Nc1cccc(n1)-c1ccc(CCN2CCN(CC(=O)c3ccccc3)CC2)cc1 Show InChI InChI=1S/C25H28N4O/c26-25-8-4-7-23(27-25)21-11-9-20(10-12-21)13-14-28-15-17-29(18-16-28)19-24(30)22-5-2-1-3-6-22/h1-12H,13-19H2,(H2,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141080
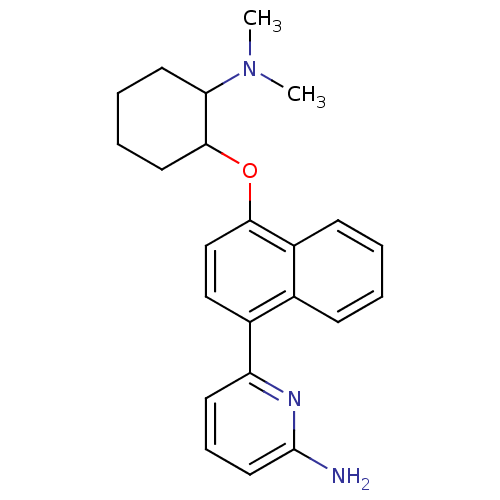
(6-[4-(2-Dimethylamino-cyclohexyloxy)-naphthalen-1-...)Show InChI InChI=1S/C23H27N3O/c1-26(2)20-11-5-6-12-22(20)27-21-15-14-17(16-8-3-4-9-18(16)21)19-10-7-13-23(24)25-19/h3-4,7-10,13-15,20,22H,5-6,11-12H2,1-2H3,(H2,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50081082

(2-(4-{2-[4-(6-Amino-pyridin-2-yl)-phenyl]-ethyl}-p...)Show SMILES Nc1cccc(n1)-c1ccc(CCN2CCN(CC(=O)c3ccccc3)CC2)cc1 Show InChI InChI=1S/C25H28N4O/c26-25-8-4-7-23(27-25)21-11-9-20(10-12-21)13-14-28-15-17-29(18-16-28)19-24(30)22-5-2-1-3-6-22/h1-12H,13-19H2,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50141085

(6-[4-(2-Pyrrolidin-1-yl-ethoxy)-5,6,7,8-tetrahydro...)Show InChI InChI=1S/C21H27N3O/c22-21-9-5-8-19(23-21)17-10-11-20(18-7-2-1-6-16(17)18)25-15-14-24-12-3-4-13-24/h5,8-11H,1-4,6-7,12-15H2,(H2,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50141080

(6-[4-(2-Dimethylamino-cyclohexyloxy)-naphthalen-1-...)Show InChI InChI=1S/C23H27N3O/c1-26(2)20-11-5-6-12-22(20)27-21-15-14-17(16-8-3-4-9-18(16)21)19-10-7-13-23(24)25-19/h3-4,7-10,13-15,20,22H,5-6,11-12H2,1-2H3,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141084
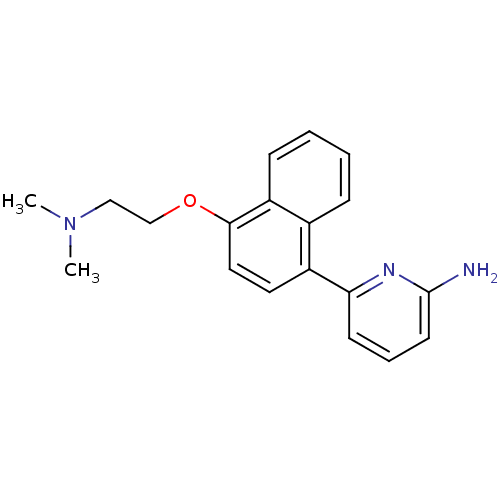
(6-[4-(2-Dimethylamino-ethoxy)-naphthalen-1-yl]-pyr...)Show InChI InChI=1S/C19H21N3O/c1-22(2)12-13-23-18-11-10-15(14-6-3-4-7-16(14)18)17-8-5-9-19(20)21-17/h3-11H,12-13H2,1-2H3,(H2,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141076
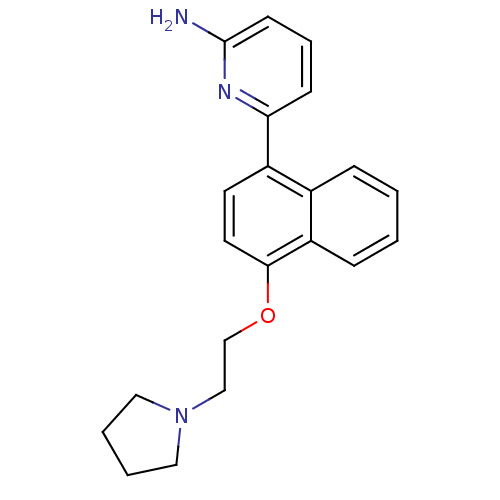
(6-[4-(2-Pyrrolidin-1-yl-ethoxy)-naphthalen-1-yl]-p...)Show InChI InChI=1S/C21H23N3O/c22-21-9-5-8-19(23-21)17-10-11-20(18-7-2-1-6-16(17)18)25-15-14-24-12-3-4-13-24/h1-2,5-11H,3-4,12-15H2,(H2,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141075

(6-[8-(2-Dimethylamino-ethoxy)-1,2,3,4-tetrahydro-1...)Show InChI InChI=1S/C20H25N3O/c1-23(2)10-11-24-17-9-8-15(16-4-3-5-18(21)22-16)19-13-6-7-14(12-13)20(17)19/h3-5,8-9,13-14H,6-7,10-12H2,1-2H3,(H2,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141087

(6-{4-[2-(4-Dimethylamino-piperidin-1-yl)-ethoxy]-n...)Show SMILES CN(C)C1CCN(CCOc2ccc(-c3cccc(N)n3)c3ccccc23)CC1 Show InChI InChI=1S/C24H30N4O/c1-27(2)18-12-14-28(15-13-18)16-17-29-23-11-10-20(19-6-3-4-7-21(19)23)22-8-5-9-24(25)26-22/h3-11,18H,12-17H2,1-2H3,(H2,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50141087

(6-{4-[2-(4-Dimethylamino-piperidin-1-yl)-ethoxy]-n...)Show SMILES CN(C)C1CCN(CCOc2ccc(-c3cccc(N)n3)c3ccccc23)CC1 Show InChI InChI=1S/C24H30N4O/c1-27(2)18-12-14-28(15-13-18)16-17-29-23-11-10-20(19-6-3-4-7-21(19)23)22-8-5-9-24(25)26-22/h3-11,18H,12-17H2,1-2H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50141084

(6-[4-(2-Dimethylamino-ethoxy)-naphthalen-1-yl]-pyr...)Show InChI InChI=1S/C19H21N3O/c1-22(2)12-13-23-18-11-10-15(14-6-3-4-7-16(14)18)17-8-5-9-19(20)21-17/h3-11H,12-13H2,1-2H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50141076

(6-[4-(2-Pyrrolidin-1-yl-ethoxy)-naphthalen-1-yl]-p...)Show InChI InChI=1S/C21H23N3O/c22-21-9-5-8-19(23-21)17-10-11-20(18-7-2-1-6-16(17)18)25-15-14-24-12-3-4-13-24/h1-2,5-11H,3-4,12-15H2,(H2,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50141078
(6-{4-[2-(4-Methyl-piperazin-1-yl)-ethoxy]-naphthal...)Show InChI InChI=1S/C22H26N4O/c1-25-11-13-26(14-12-25)15-16-27-21-10-9-18(17-5-2-3-6-19(17)21)20-7-4-8-22(23)24-20/h2-10H,11-16H2,1H3,(H2,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 306 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50141085

(6-[4-(2-Pyrrolidin-1-yl-ethoxy)-5,6,7,8-tetrahydro...)Show InChI InChI=1S/C21H27N3O/c22-21-9-5-8-19(23-21)17-10-11-20(18-7-2-1-6-16(17)18)25-15-14-24-12-3-4-13-24/h5,8-11H,1-4,6-7,12-15H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 358 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human inducible nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50141078

(6-{4-[2-(4-Methyl-piperazin-1-yl)-ethoxy]-naphthal...)Show InChI InChI=1S/C22H26N4O/c1-25-11-13-26(14-12-25)15-16-27-21-10-9-18(17-5-2-3-6-19(17)21)20-7-4-8-22(23)24-20/h2-10H,11-16H2,1H3,(H2,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50141089

(6-[7-(2-Pyrrolidin-1-yl-ethoxy)-indan-4-yl]-pyridi...)Show InChI InChI=1S/C20H25N3O/c21-20-8-4-7-18(22-20)16-9-10-19(17-6-3-5-15(16)17)24-14-13-23-11-1-2-12-23/h4,7-10H,1-3,5-6,11-14H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human inducible nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50141082

(6-[4-(2-Dimethylamino-ethoxy)-5,6,7,8-tetrahydro-n...)Show InChI InChI=1S/C19H25N3O/c1-22(2)12-13-23-18-11-10-15(14-6-3-4-7-16(14)18)17-8-5-9-19(20)21-17/h5,8-11H,3-4,6-7,12-13H2,1-2H3,(H2,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 517 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human inducible nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50141086

(6-[7-(2-Dimethylamino-ethoxy)-indan-4-yl]-pyridin-...)Show InChI InChI=1S/C18H23N3O/c1-21(2)11-12-22-17-10-9-14(13-5-3-6-15(13)17)16-7-4-8-18(19)20-16/h4,7-10H,3,5-6,11-12H2,1-2H3,(H2,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 592 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human inducible nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50141089

(6-[7-(2-Pyrrolidin-1-yl-ethoxy)-indan-4-yl]-pyridi...)Show InChI InChI=1S/C20H25N3O/c21-20-8-4-7-18(22-20)16-9-10-19(17-6-3-5-15(16)17)24-14-13-23-11-1-2-12-23/h4,7-10H,1-3,5-6,11-14H2,(H2,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 785 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50081082

(2-(4-{2-[4-(6-Amino-pyridin-2-yl)-phenyl]-ethyl}-p...)Show SMILES Nc1cccc(n1)-c1ccc(CCN2CCN(CC(=O)c3ccccc3)CC2)cc1 Show InChI InChI=1S/C25H28N4O/c26-25-8-4-7-23(27-25)21-11-9-20(10-12-21)13-14-28-15-17-29(18-16-28)19-24(30)22-5-2-1-3-6-22/h1-12H,13-19H2,(H2,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 887 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50141075

(6-[8-(2-Dimethylamino-ethoxy)-1,2,3,4-tetrahydro-1...)Show InChI InChI=1S/C20H25N3O/c1-23(2)10-11-24-17-9-8-15(16-4-3-5-18(21)22-16)19-13-6-7-14(12-13)20(17)19/h3-5,8-9,13-14H,6-7,10-12H2,1-2H3,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human inducible nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50141083

(6-{4-[2-(4-Methyl-piperazin-1-yl)-ethoxy]-5,6,7,8-...)Show InChI InChI=1S/C22H30N4O/c1-25-11-13-26(14-12-25)15-16-27-21-10-9-18(17-5-2-3-6-19(17)21)20-7-4-8-22(23)24-20/h4,7-10H,2-3,5-6,11-16H2,1H3,(H2,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human inducible nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50141086

(6-[7-(2-Dimethylamino-ethoxy)-indan-4-yl]-pyridin-...)Show InChI InChI=1S/C18H23N3O/c1-21(2)11-12-22-17-10-9-14(13-5-3-6-15(13)17)16-7-4-8-18(19)20-16/h4,7-10H,3,5-6,11-12H2,1-2H3,(H2,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50141076

(6-[4-(2-Pyrrolidin-1-yl-ethoxy)-naphthalen-1-yl]-p...)Show InChI InChI=1S/C21H23N3O/c22-21-9-5-8-19(23-21)17-10-11-20(18-7-2-1-6-16(17)18)25-15-14-24-12-3-4-13-24/h1-2,5-11H,3-4,12-15H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human inducible nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50141081

(6-[4-(1-Methyl-pyrrolidin-3-yloxy)-naphthalen-1-yl...)Show InChI InChI=1S/C20H21N3O/c1-23-12-11-14(13-23)24-19-10-9-16(15-5-2-3-6-17(15)19)18-7-4-8-20(21)22-18/h2-10,14H,11-13H2,1H3,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human inducible nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50141088

(6-[4-(2-Dimethylamino-ethoxy)-6,7,8,9-tetrahydro-5...)Show InChI InChI=1S/C20H27N3O/c1-23(2)13-14-24-19-12-11-16(18-9-6-10-20(21)22-18)15-7-4-3-5-8-17(15)19/h6,9-12H,3-5,7-8,13-14H2,1-2H3,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human inducible nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50141074

(6-{7-[2-(4-Methyl-piperazin-1-yl)-ethoxy]-indan-4-...)Show InChI InChI=1S/C21H28N4O/c1-24-10-12-25(13-11-24)14-15-26-20-9-8-17(16-4-2-5-18(16)20)19-6-3-7-21(22)23-19/h3,6-9H,2,4-5,10-15H2,1H3,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human inducible nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50141085

(6-[4-(2-Pyrrolidin-1-yl-ethoxy)-5,6,7,8-tetrahydro...)Show InChI InChI=1S/C21H27N3O/c22-21-9-5-8-19(23-21)17-10-11-20(18-7-2-1-6-16(17)18)25-15-14-24-12-3-4-13-24/h5,8-11H,1-4,6-7,12-15H2,(H2,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50141080

(6-[4-(2-Dimethylamino-cyclohexyloxy)-naphthalen-1-...)Show InChI InChI=1S/C23H27N3O/c1-26(2)20-11-5-6-12-22(20)27-21-15-14-17(16-8-3-4-9-18(16)21)19-10-7-13-23(24)25-19/h3-4,7-10,13-15,20,22H,5-6,11-12H2,1-2H3,(H2,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human inducible nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50209245

(CHEMBL247378)Show InChI InChI=1S/C7H5N3O2/c11-10(12)6-3-1-2-5-4-8-9-7(5)6/h1-4H,(H,8,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50209245

(CHEMBL247378)Show InChI InChI=1S/C7H5N3O2/c11-10(12)6-3-1-2-5-4-8-9-7(5)6/h1-4H,(H,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neuronal nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50141077

(6-[4-(1-Methyl-pyrrolidin-2-ylmethoxy)-naphthalen-...)Show InChI InChI=1S/C21H23N3O/c1-24-13-5-6-15(24)14-25-20-12-11-17(16-7-2-3-8-18(16)20)19-9-4-10-21(22)23-19/h2-4,7-12,15H,5-6,13-14H2,1H3,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human inducible nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50141077

(6-[4-(1-Methyl-pyrrolidin-2-ylmethoxy)-naphthalen-...)Show InChI InChI=1S/C21H23N3O/c1-24-13-5-6-15(24)14-25-20-12-11-17(16-7-2-3-8-18(16)20)19-9-4-10-21(22)23-19/h2-4,7-12,15H,5-6,13-14H2,1H3,(H2,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50141074

(6-{7-[2-(4-Methyl-piperazin-1-yl)-ethoxy]-indan-4-...)Show InChI InChI=1S/C21H28N4O/c1-24-10-12-25(13-11-24)14-15-26-20-9-8-17(16-4-2-5-18(16)20)19-6-3-7-21(22)23-19/h3,6-9H,2,4-5,10-15H2,1H3,(H2,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50141082

(6-[4-(2-Dimethylamino-ethoxy)-5,6,7,8-tetrahydro-n...)Show InChI InChI=1S/C19H25N3O/c1-22(2)12-13-23-18-11-10-15(14-6-3-4-7-16(14)18)17-8-5-9-19(20)21-17/h5,8-11H,3-4,6-7,12-13H2,1-2H3,(H2,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50141076

(6-[4-(2-Pyrrolidin-1-yl-ethoxy)-naphthalen-1-yl]-p...)Show InChI InChI=1S/C21H23N3O/c22-21-9-5-8-19(23-21)17-10-11-20(18-7-2-1-6-16(17)18)25-15-14-24-12-3-4-13-24/h1-2,5-11H,3-4,12-15H2,(H2,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50141079

(6-[4-(1-Aza-bicyclo[2.2.2]oct-2-ylmethoxy)-naphtha...)Show SMILES Nc1cccc(n1)-c1ccc(OCC2CC3CCN2CC3)c2ccccc12 |(11.24,-2.54,;9.89,-1.75,;9.89,-.21,;8.56,.56,;7.23,-.21,;7.23,-1.75,;8.56,-2.52,;5.89,-2.52,;4.57,-1.75,;3.24,-2.52,;3.24,-4.06,;1.89,-4.85,;.57,-4.08,;-.76,-4.86,;-2.09,-4.08,;-3.43,-4.85,;-1.9,-4.84,;-2.3,-6.31,;-.76,-6.4,;-2.11,-7.17,;-3.44,-6.4,;4.57,-4.82,;4.57,-6.37,;5.89,-7.14,;7.23,-6.37,;7.23,-4.82,;5.89,-4.06,)| Show InChI InChI=1S/C23H25N3O/c24-23-7-3-6-21(25-23)19-8-9-22(20-5-2-1-4-18(19)20)27-15-17-14-16-10-12-26(17)13-11-16/h1-9,16-17H,10-15H2,(H2,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50141084

(6-[4-(2-Dimethylamino-ethoxy)-naphthalen-1-yl]-pyr...)Show InChI InChI=1S/C19H21N3O/c1-22(2)12-13-23-18-11-10-15(14-6-3-4-7-16(14)18)17-8-5-9-19(20)21-17/h3-11H,12-13H2,1-2H3,(H2,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human inducible nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50141088

(6-[4-(2-Dimethylamino-ethoxy)-6,7,8,9-tetrahydro-5...)Show InChI InChI=1S/C20H27N3O/c1-23(2)13-14-24-19-12-11-16(18-9-6-10-20(21)22-18)15-7-4-3-5-8-17(15)19/h6,9-12H,3-5,7-8,13-14H2,1-2H3,(H2,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50141087

(6-{4-[2-(4-Dimethylamino-piperidin-1-yl)-ethoxy]-n...)Show SMILES CN(C)C1CCN(CCOc2ccc(-c3cccc(N)n3)c3ccccc23)CC1 Show InChI InChI=1S/C24H30N4O/c1-27(2)18-12-14-28(15-13-18)16-17-29-23-11-10-20(19-6-3-4-7-21(19)23)22-8-5-9-24(25)26-22/h3-11,18H,12-17H2,1-2H3,(H2,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human inducible nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50141084

(6-[4-(2-Dimethylamino-ethoxy)-naphthalen-1-yl]-pyr...)Show InChI InChI=1S/C19H21N3O/c1-22(2)12-13-23-18-11-10-15(14-6-3-4-7-16(14)18)17-8-5-9-19(20)21-17/h3-11H,12-13H2,1-2H3,(H2,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50141079

(6-[4-(1-Aza-bicyclo[2.2.2]oct-2-ylmethoxy)-naphtha...)Show SMILES Nc1cccc(n1)-c1ccc(OCC2CC3CCN2CC3)c2ccccc12 |(11.24,-2.54,;9.89,-1.75,;9.89,-.21,;8.56,.56,;7.23,-.21,;7.23,-1.75,;8.56,-2.52,;5.89,-2.52,;4.57,-1.75,;3.24,-2.52,;3.24,-4.06,;1.89,-4.85,;.57,-4.08,;-.76,-4.86,;-2.09,-4.08,;-3.43,-4.85,;-1.9,-4.84,;-2.3,-6.31,;-.76,-6.4,;-2.11,-7.17,;-3.44,-6.4,;4.57,-4.82,;4.57,-6.37,;5.89,-7.14,;7.23,-6.37,;7.23,-4.82,;5.89,-4.06,)| Show InChI InChI=1S/C23H25N3O/c24-23-7-3-6-21(25-23)19-8-9-22(20-5-2-1-4-18(19)20)27-15-17-14-16-10-12-26(17)13-11-16/h1-9,16-17H,10-15H2,(H2,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human inducible nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50141087

(6-{4-[2-(4-Dimethylamino-piperidin-1-yl)-ethoxy]-n...)Show SMILES CN(C)C1CCN(CCOc2ccc(-c3cccc(N)n3)c3ccccc23)CC1 Show InChI InChI=1S/C24H30N4O/c1-27(2)18-12-14-28(15-13-18)16-17-29-23-11-10-20(19-6-3-4-7-21(19)23)22-8-5-9-24(25)26-22/h3-11,18H,12-17H2,1-2H3,(H2,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50141078

(6-{4-[2-(4-Methyl-piperazin-1-yl)-ethoxy]-naphthal...)Show InChI InChI=1S/C22H26N4O/c1-25-11-13-26(14-12-25)15-16-27-21-10-9-18(17-5-2-3-6-19(17)21)20-7-4-8-22(23)24-20/h2-10H,11-16H2,1H3,(H2,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human inducible nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50141083

(6-{4-[2-(4-Methyl-piperazin-1-yl)-ethoxy]-5,6,7,8-...)Show InChI InChI=1S/C22H30N4O/c1-25-11-13-26(14-12-25)15-16-27-21-10-9-18(17-5-2-3-6-19(17)21)20-7-4-8-22(23)24-20/h4,7-10H,2-3,5-6,11-16H2,1H3,(H2,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50141078

(6-{4-[2-(4-Methyl-piperazin-1-yl)-ethoxy]-naphthal...)Show InChI InChI=1S/C22H26N4O/c1-25-11-13-26(14-12-25)15-16-27-21-10-9-18(17-5-2-3-6-19(17)21)20-7-4-8-22(23)24-20/h2-10H,11-16H2,1H3,(H2,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50141080

(6-[4-(2-Dimethylamino-cyclohexyloxy)-naphthalen-1-...)Show InChI InChI=1S/C23H27N3O/c1-26(2)20-11-5-6-12-22(20)27-21-15-14-17(16-8-3-4-9-18(16)21)19-10-7-13-23(24)25-19/h3-4,7-10,13-15,20,22H,5-6,11-12H2,1-2H3,(H2,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50141081

(6-[4-(1-Methyl-pyrrolidin-3-yloxy)-naphthalen-1-yl...)Show InChI InChI=1S/C20H21N3O/c1-23-12-11-14(13-23)24-19-10-9-16(15-5-2-3-6-17(15)19)18-7-4-8-20(21)22-18/h2-10,14H,11-13H2,1H3,(H2,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50141075

(6-[8-(2-Dimethylamino-ethoxy)-1,2,3,4-tetrahydro-1...)Show InChI InChI=1S/C20H25N3O/c1-23(2)10-11-24-17-9-8-15(16-4-3-5-18(21)22-16)19-13-6-7-14(12-13)20(17)19/h3-5,8-9,13-14H,6-7,10-12H2,1-2H3,(H2,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human endothelial nitric oxide synthase |
J Med Chem 47: 1575-86 (2004)
Article DOI: 10.1021/jm030519g
BindingDB Entry DOI: 10.7270/Q2J102MT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data