

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50004679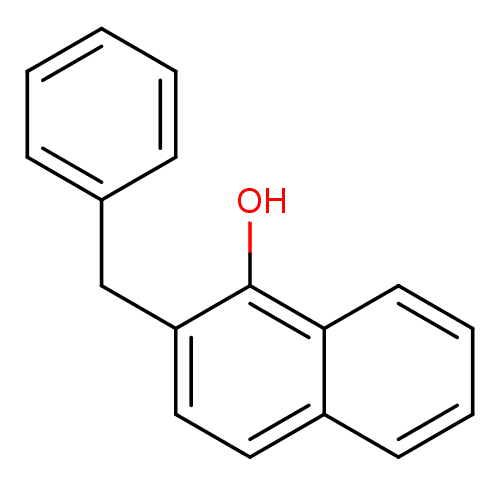 (2-Benzyl-naphthalen-1-ol | 2-Benzyl-naphthalen-1-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 5-lipoxygenase of RBL-1 cell line | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of Bodipy-labelled cyclopamine from Smo expressed in COS-1 cells in presence of 2% FBS after 4 to 6 hrs by FACS flow cytometric analysis | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50004677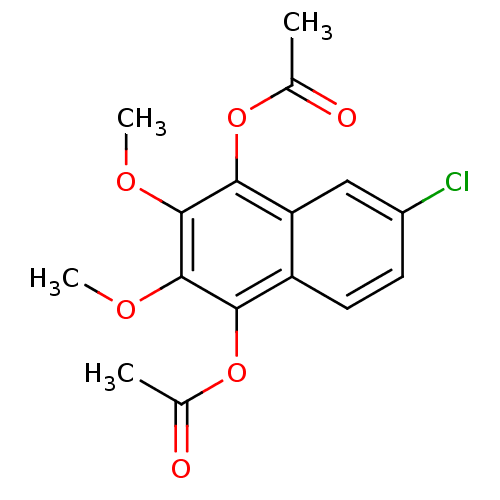 (Acetic acid 4-acetoxy-6-chloro-2,3-dimethoxy-napht...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 5-lipoxygenase of RBL-1 cell line | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50004678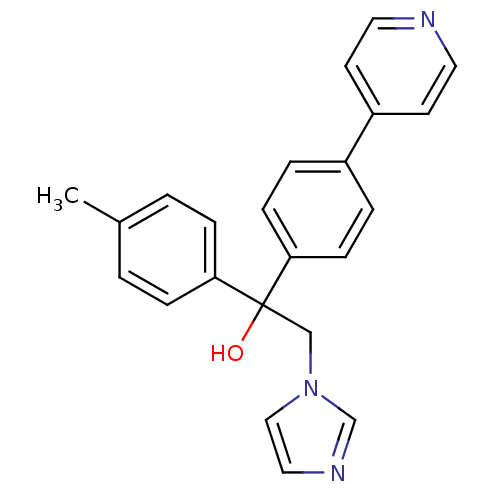 (2-Imidazol-1-yl-1-(4-pyridin-4-yl-phenyl)-1-p-toly...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 5-lipoxygenase of RBL-1 cell line | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 5-lipoxygenase of RBL-1 cell line | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM31768 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 5-lipoxygenase of RBL-1 cell line | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50004676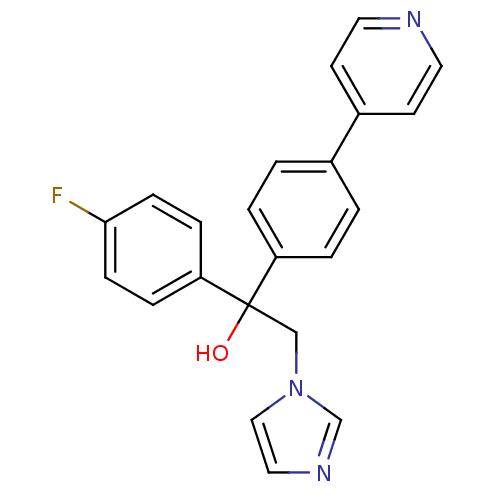 (1-(4-Fluoro-phenyl)-2-imidazol-1-yl-1-(4-pyridin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against 5-lipoxygenase of RBL-1 cell line | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50004679 (2-Benzyl-naphthalen-1-ol | 2-Benzyl-naphthalen-1-o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >7.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of Bodipy-labelled cyclopamine from Smo expressed in COS-1 cells in presence of 2% FBS after 4 to 6 hrs by FACS flow cytometric analysis | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50004677 (Acetic acid 4-acetoxy-6-chloro-2,3-dimethoxy-napht...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >7.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of Bodipy-labelled cyclopamine from Smo expressed in COS-1 cells in presence of 2% FBS after 4 to 6 hrs by FACS flow cytometric analysis | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50004678 (2-Imidazol-1-yl-1-(4-pyridin-4-yl-phenyl)-1-p-toly...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >7.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of Bodipy-labelled cyclopamine from Smo expressed in COS-1 cells in presence of 2% FBS after 4 to 6 hrs by FACS flow cytometric analysis | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM31768 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >7.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of Bodipy-labelled cyclopamine from Smo expressed in COS-1 cells in presence of 2% FBS after 4 to 6 hrs by FACS flow cytometric analysis | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Bos taurus) | BDBM50004676 (1-(4-Fluoro-phenyl)-2-imidazol-1-yl-1-(4-pyridin-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >7.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Displacement of Bodipy-labelled cyclopamine from Smo expressed in COS-1 cells in presence of 2% FBS after 4 to 6 hrs by FACS flow cytometric analysis | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, major isoenzyme (Sus scrofa (pig)) | BDBM31768 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against phospholipase A2 of Croatalus adamanteus | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, major isoenzyme (Sus scrofa (pig)) | BDBM50004677 (Acetic acid 4-acetoxy-6-chloro-2,3-dimethoxy-napht...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against phospholipase A2 of Croatalus adamanteus | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, major isoenzyme (Sus scrofa (pig)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against phospholipase A2 of Croatalus adamanteus | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, major isoenzyme (Sus scrofa (pig)) | BDBM50004676 (1-(4-Fluoro-phenyl)-2-imidazol-1-yl-1-(4-pyridin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against phospholipase A2 of Croatalus adamanteus | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, major isoenzyme (Sus scrofa (pig)) | BDBM50004678 (2-Imidazol-1-yl-1-(4-pyridin-4-yl-phenyl)-1-p-toly...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against phospholipase A2 of Croatalus adamanteus | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, major isoenzyme (Sus scrofa (pig)) | BDBM50004679 (2-Benzyl-naphthalen-1-ol | 2-Benzyl-naphthalen-1-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound against phospholipase A2 of Croatalus adamanteus | J Med Chem 35: 3148-55 (1992) BindingDB Entry DOI: 10.7270/Q2V69HJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||