

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50005321 (5-[2-(2-Acetylamino-3-phenyl-propionylamino)-hexan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human plasma renin at pH 7.4 | J Med Chem 35: 833-46 (1992) BindingDB Entry DOI: 10.7270/Q21R6PGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005318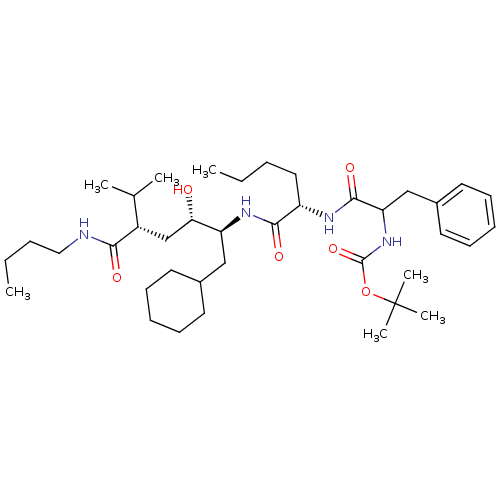 (CHEMBL166771 | {1-[1-(4-Butylcarbamoyl-1-cyclohexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human plasma renin at pH 7.4 | J Med Chem 35: 833-46 (1992) BindingDB Entry DOI: 10.7270/Q21R6PGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005312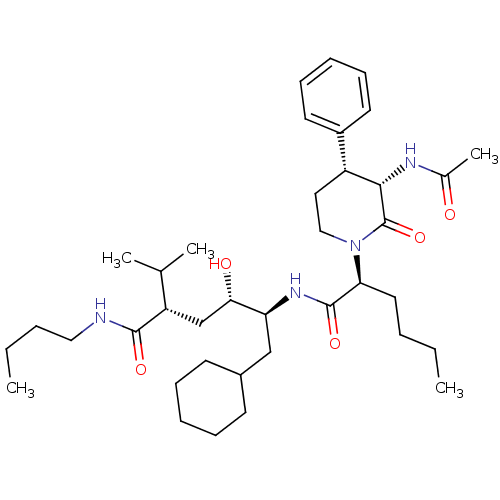 (5-[2-(3-Acetylamino-2-oxo-4-phenyl-piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human plasma renin at pH 7.4 | J Med Chem 35: 833-46 (1992) BindingDB Entry DOI: 10.7270/Q21R6PGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005319 (6-Cyclohexyl-5-{2-[2-(1,3-dioxo-1,3-dihydro-isoind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human plasma renin at pH 7.4 | J Med Chem 35: 833-46 (1992) BindingDB Entry DOI: 10.7270/Q21R6PGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005316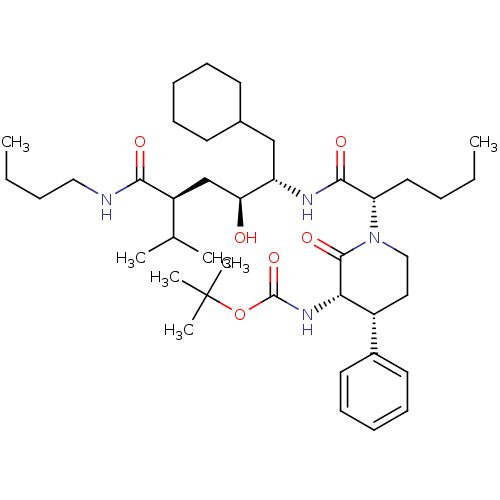 (CHEMBL350357 | {1-[1-(4-Butylcarbamoyl-1-cyclohexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 134 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human plasma renin at pH 7.4 | J Med Chem 35: 833-46 (1992) BindingDB Entry DOI: 10.7270/Q21R6PGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005315 (5-[2-(4-Acetylamino-3-oxo-1,3,4,5-tetrahydro-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human plasma renin at pH 7.4 | J Med Chem 35: 833-46 (1992) BindingDB Entry DOI: 10.7270/Q21R6PGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005313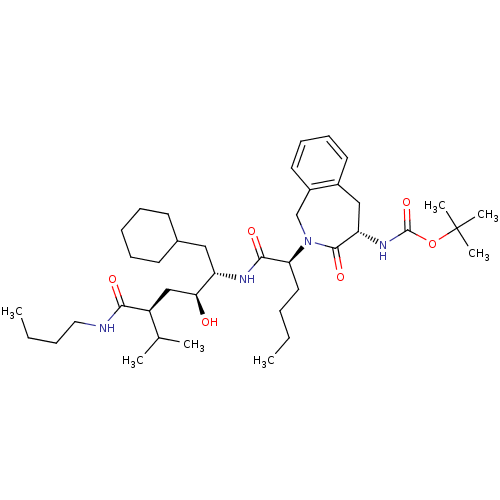 (CHEMBL169718 | {2-[1-(4-Butylcarbamoyl-1-cyclohexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human plasma renin at pH 7.4 | J Med Chem 35: 833-46 (1992) BindingDB Entry DOI: 10.7270/Q21R6PGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005317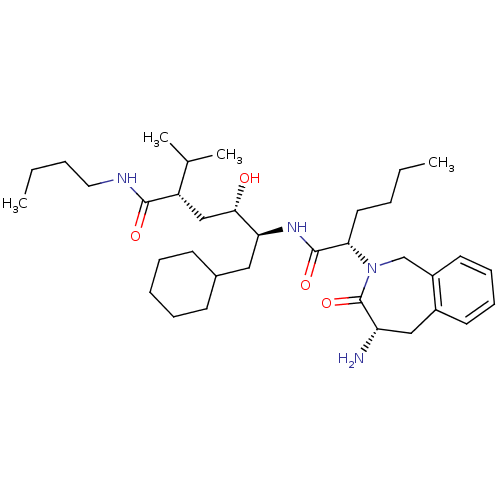 (5-[2-(4-Amino-3-oxo-1,3,4,5-tetrahydro-benzo[c]aze...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human plasma renin at pH 7.4 | J Med Chem 35: 833-46 (1992) BindingDB Entry DOI: 10.7270/Q21R6PGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005314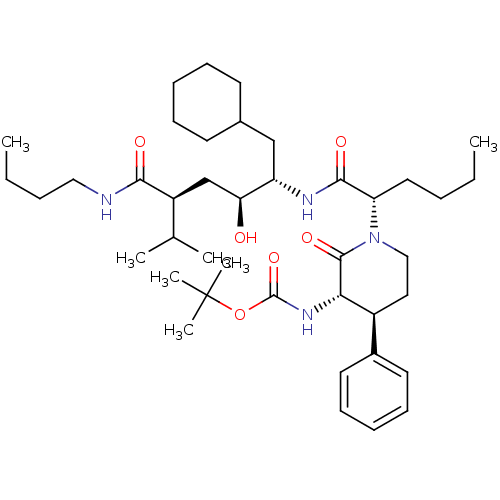 (CHEMBL167118 | {1-[1-(4-Butylcarbamoyl-1-cyclohexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.91E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human plasma renin at pH 7.4 | J Med Chem 35: 833-46 (1992) BindingDB Entry DOI: 10.7270/Q21R6PGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005320 (6-Cyclohexyl-5-{2-[4-(1,3-dioxo-1,3-dihydro-isoind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human plasma renin at pH 7.4 | J Med Chem 35: 833-46 (1992) BindingDB Entry DOI: 10.7270/Q21R6PGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||