

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50005417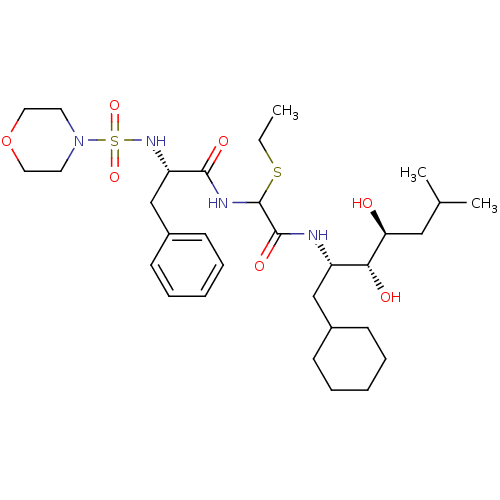 (CHEMBL266334 | N-[(1-Cyclohexylmethyl-2,3-dihydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005437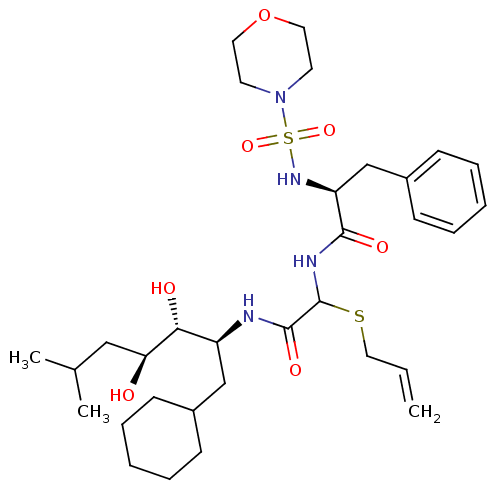 (CHEMBL8477 | N-[Allylsulfanyl-(1-cyclohexylmethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005443 (CHEMBL441325 | N-[(1-Cyclohexylmethyl-2,3-dihydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005431 (CHEMBL8665 | N-[(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005442 (CHEMBL262712 | N-[Allyloxy-(1-cyclohexylmethyl-2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005445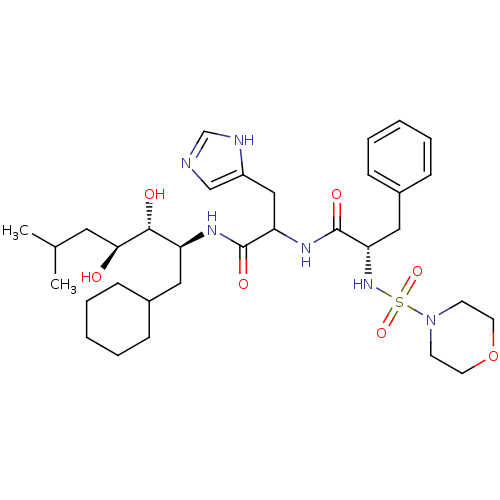 (CHEMBL267277 | N-[1-(1-Cyclohexylmethyl-2,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005428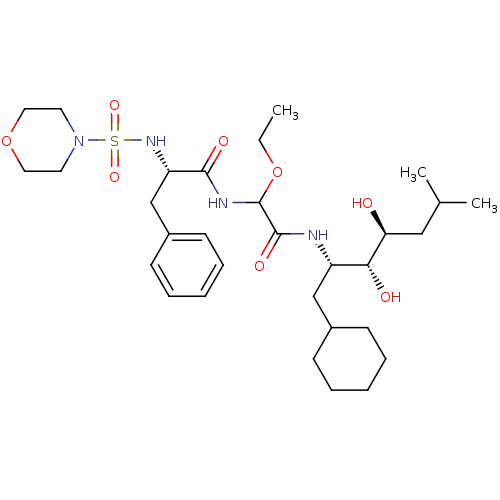 (CHEMBL8836 | N-[(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005422 (5-Cyclohexyl-2,2-difluoro-3-hydroxy-4-{2-[2-(morph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005423 (CHEMBL266139 | N-[(1-Cyclohexylmethyl-2,3-dihydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005439 ((1-{[(1-Cyclohexylmethyl-2,3-dihydroxy-5-methyl-he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005436 (4-{2-Allylsulfanyl-2-[2-(morpholine-4-sulfonylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005433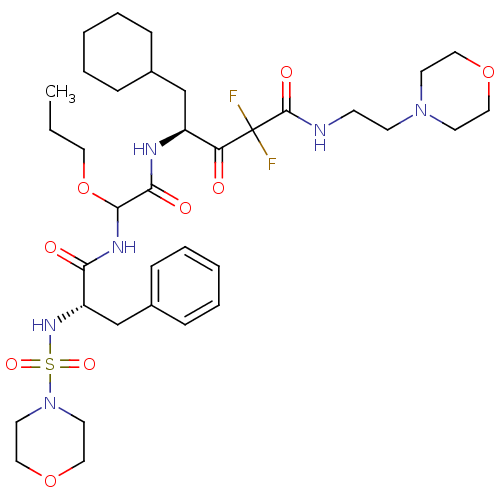 (5-Cyclohexyl-2,2-difluoro-4-{2-[2-(morpholine-4-su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005420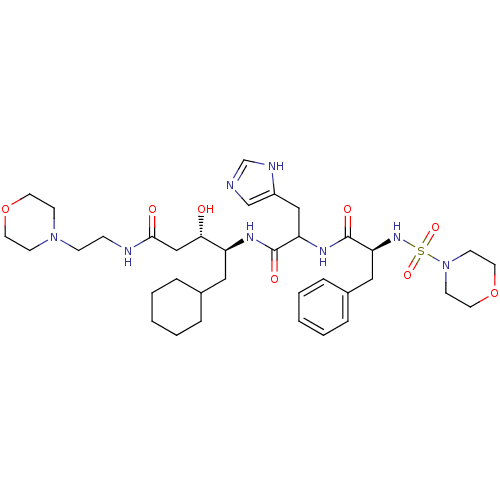 (5-Cyclohexyl-3-hydroxy-4-{3-(1H-imidazol-4-yl)-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005418 (5-Cyclohexyl-4-{2-ethoxy-2-[2-(morpholine-4-sulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50005417 (CHEMBL266334 | N-[(1-Cyclohexylmethyl-2,3-dihydrox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against bovine cathepsin D | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50005436 (4-{2-Allylsulfanyl-2-[2-(morpholine-4-sulfonylamin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against bovine cathepsin D | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50005442 (CHEMBL262712 | N-[Allyloxy-(1-cyclohexylmethyl-2,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against bovine cathepsin D | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005444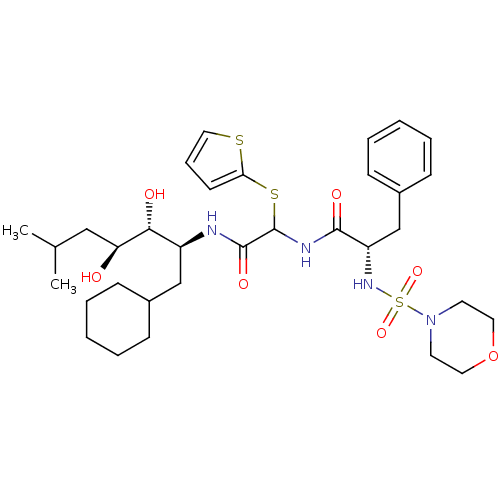 (CHEMBL406692 | N-[(1-Cyclohexylmethyl-2,3-dihydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50005428 (CHEMBL8836 | N-[(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against bovine cathepsin D | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50005437 (CHEMBL8477 | N-[Allylsulfanyl-(1-cyclohexylmethyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against bovine cathepsin D | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50005443 (CHEMBL441325 | N-[(1-Cyclohexylmethyl-2,3-dihydrox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against bovine cathepsin D | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50005431 (CHEMBL8665 | N-[(1-Cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against bovine cathepsin D | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005427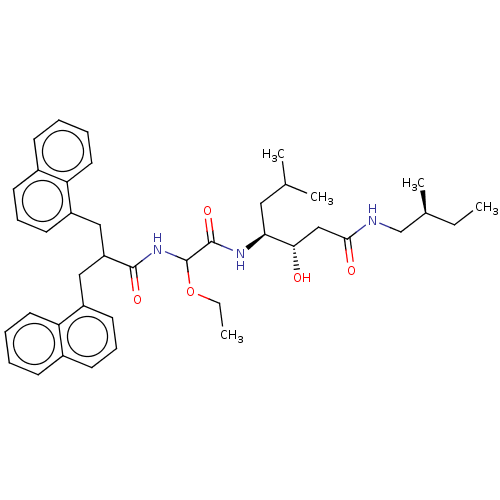 (4-[2-Ethoxy-2-(3-naphthalen-1-yl-2-naphthalen-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50005439 ((1-{[(1-Cyclohexylmethyl-2,3-dihydroxy-5-methyl-he...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against bovine cathepsin D | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005425 ((1-{[(Allyl-methyl-amino)-(1-cyclohexylmethyl-2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50005425 ((1-{[(Allyl-methyl-amino)-(1-cyclohexylmethyl-2,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against bovine cathepsin D | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005430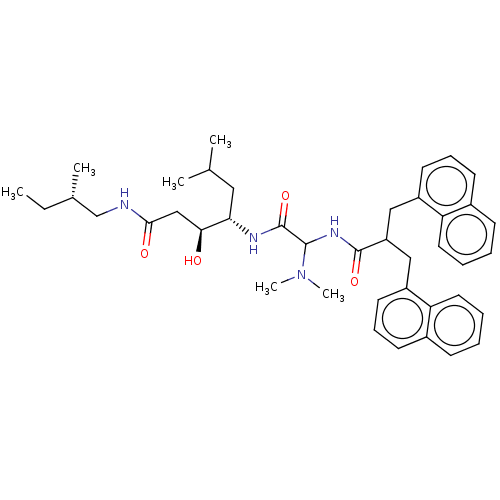 (4-[2-Dimethylamino-2-(3-naphthalen-1-yl-2-naphthal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50005423 (CHEMBL266139 | N-[(1-Cyclohexylmethyl-2,3-dihydrox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against bovine cathepsin D | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005416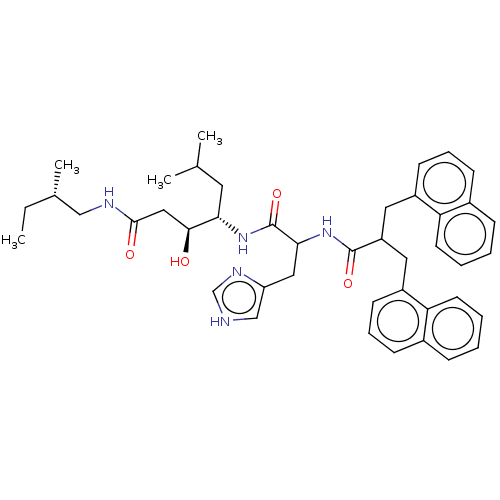 (3-Hydroxy-4-[3-(1H-imidazol-4-yl)-2-(3-naphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005438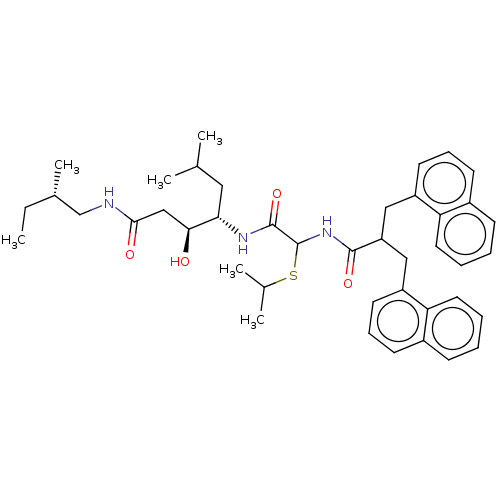 (3-Hydroxy-4-[2-isopropylsulfanyl-2-(3-naphthalen-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005419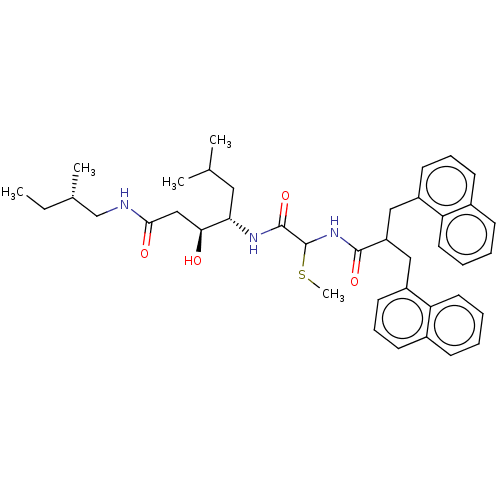 (3-Hydroxy-6-methyl-4-[2-methylsulfanyl-2-(3-naphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50005433 (5-Cyclohexyl-2,2-difluoro-4-{2-[2-(morpholine-4-su...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against bovine cathepsin D | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005434 (3-Hydroxy-4-[2-methoxy-2-(3-naphthalen-1-yl-2-naph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005426 (4-[2-Ethylamino-2-(3-naphthalen-1-yl-2-naphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005441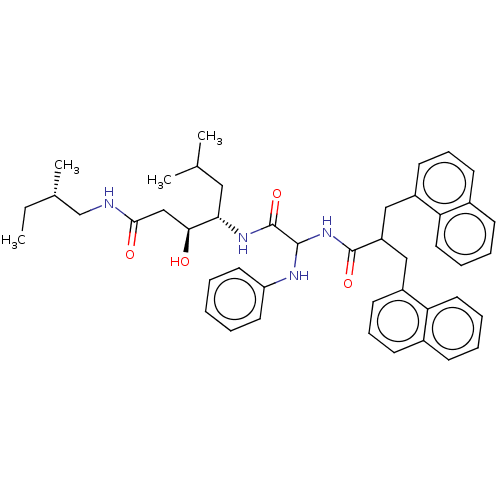 (3-Hydroxy-6-methyl-4-[2-(3-naphthalen-1-yl-2-napht...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50005444 (CHEMBL406692 | N-[(1-Cyclohexylmethyl-2,3-dihydrox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against bovine cathepsin D | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005424 (3-Hydroxy-4-[2-isopropylamino-2-(3-naphthalen-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005432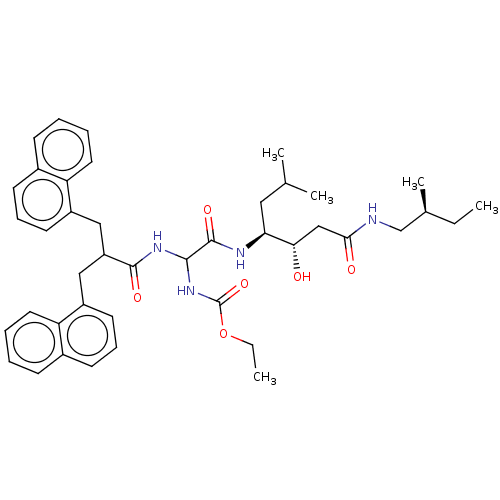 (CHEMBL3350031 | [{1-[1-Hydroxy-2-(2-methyl-butylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 516 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50230581 (CHEMBL3350033) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 615 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005440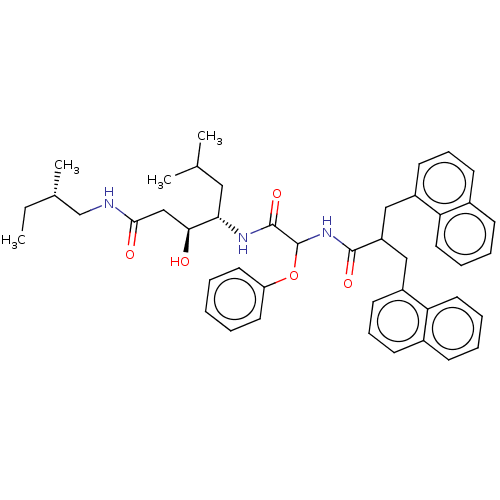 (3-Hydroxy-6-methyl-4-[2-(3-naphthalen-1-yl-2-napht...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 633 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005421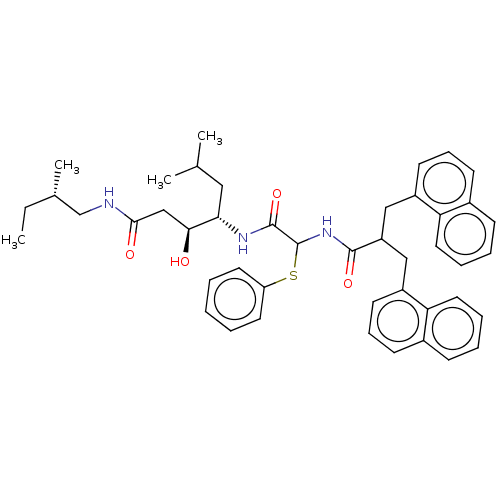 (3-Hydroxy-6-methyl-4-[2-(3-naphthalen-1-yl-2-napht...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 656 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50005422 (5-Cyclohexyl-2,2-difluoro-3-hydroxy-4-{2-[2-(morph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 699 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against bovine cathepsin D | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005447 (4-[2-Acetylamino-2-(3-naphthalen-1-yl-2-naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 936 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005435 (4-[2-Amino-2-(3-naphthalen-1-yl-2-naphthalen-1-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005435 (4-[2-Amino-2-(3-naphthalen-1-yl-2-naphthalen-1-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005446 (3-Hydroxy-4-[2-methanesulfonyl-2-(3-naphthalen-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005424 (3-Hydroxy-4-[2-isopropylamino-2-(3-naphthalen-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005427 (4-[2-Ethoxy-2-(3-naphthalen-1-yl-2-naphthalen-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005448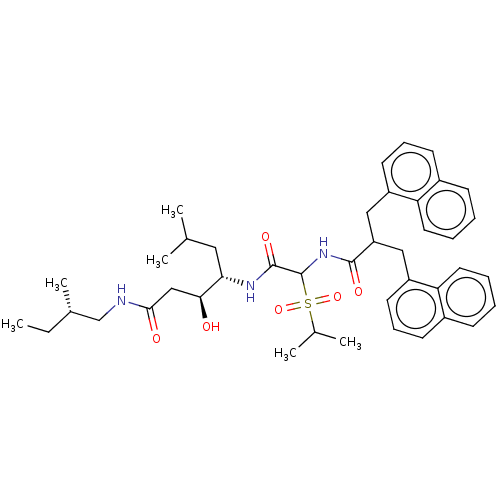 (3-Hydroxy-6-methyl-4-[2-(3-naphthalen-1-yl-2-napht...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005426 (4-[2-Ethylamino-2-(3-naphthalen-1-yl-2-naphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005430 (4-[2-Dimethylamino-2-(3-naphthalen-1-yl-2-naphthal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005438 (3-Hydroxy-4-[2-isopropylsulfanyl-2-(3-naphthalen-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against monkey renin in vitro. | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50005420 (5-Cyclohexyl-3-hydroxy-4-{3-(1H-imidazol-4-yl)-2-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against bovine cathepsin D | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50005445 (CHEMBL267277 | N-[1-(1-Cyclohexylmethyl-2,3-dihydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against bovine cathepsin D | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM50005418 (5-Cyclohexyl-4-{2-ethoxy-2-[2-(morpholine-4-sulfon...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity against bovine cathepsin D | J Med Chem 35: 1032-42 (1992) BindingDB Entry DOI: 10.7270/Q2NG4PKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||