 Found 7 hits of Enzyme Inhibition Constant Data
Found 7 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bile acid receptor
(Homo sapiens (Human)) | BDBM21674
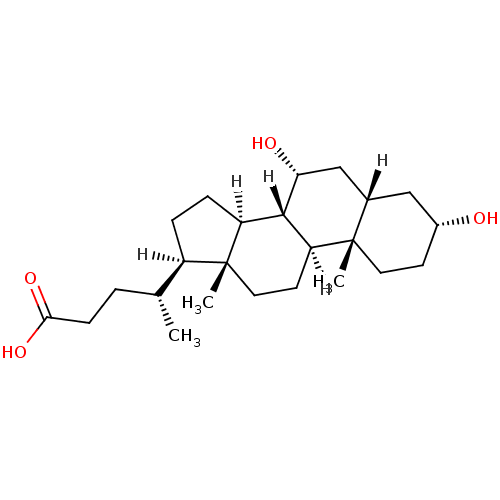
((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihyd...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20-,22+,23+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a |
Universit£ di Perugia
Curated by ChEMBL
| Assay Description
Effective concentration for recruitment of SRC-1 LxxLL-containing peptide to human Farnesoid X receptor |
J Med Chem 48: 5383-403 (2005)
Article DOI: 10.1021/jm0582221
BindingDB Entry DOI: 10.7270/Q2N29XQ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21675

((4R)-4-[(1S,2S,5R,7S,8R,9R,10S,11S,14R,15R)-8-ethy...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C26H44O4/c1-5-17-21-14-16(27)10-12-26(21,4)20-11-13-25(3)18(15(2)6-9-22(28)29)7-8-19(25)23(20)24(17)30/h15-21,23-24,27,30H,5-14H2,1-4H3,(H,28,29)/t15-,16-,17-,18-,19+,20+,21+,23+,24-,25-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 99 | n/a | n/a | n/a | n/a |
Universit£ di Perugia
Curated by ChEMBL
| Assay Description
Activation of human farnesoid X receptor |
J Med Chem 48: 5383-403 (2005)
Article DOI: 10.1021/jm0582221
BindingDB Entry DOI: 10.7270/Q2N29XQ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50171848

((E)-3-{3-[Cyclohexanecarbonyl-(4-styryl-benzyl)-am...)Show SMILES OC(=O)\C=C\c1cccc(c1)N(Cc1ccc(\C=C\c2ccccc2)cc1)C(=O)C1CCCCC1 Show InChI InChI=1S/C31H31NO3/c33-30(34)21-20-26-10-7-13-29(22-26)32(31(35)28-11-5-2-6-12-28)23-27-18-16-25(17-19-27)15-14-24-8-3-1-4-9-24/h1,3-4,7-10,13-22,28H,2,5-6,11-12,23H2,(H,33,34)/b15-14+,21-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.55E+5 | n/a | n/a | n/a | n/a |
Universit£ di Perugia
Curated by ChEMBL
| Assay Description
Activation of human farnesoid X receptor in FRET assay |
J Med Chem 48: 5383-403 (2005)
Article DOI: 10.1021/jm0582221
BindingDB Entry DOI: 10.7270/Q2N29XQ6 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50171847

((E)-3-{3-[Cyclohexanecarbonyl-(4'-dimethylamino-bi...)Show SMILES CN(C)c1ccc(cc1)-c1ccc(CN(C(=O)C2CCCCC2)c2cccc(\C=C\C(O)=O)c2)cc1 Show InChI InChI=1S/C31H34N2O3/c1-32(2)28-18-16-26(17-19-28)25-14-11-24(12-15-25)22-33(31(36)27-8-4-3-5-9-27)29-10-6-7-23(21-29)13-20-30(34)35/h6-7,10-21,27H,3-5,8-9,22H2,1-2H3,(H,34,35)/b20-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.55E+5 | n/a | n/a | n/a | n/a |
Universit£ di Perugia
Curated by ChEMBL
| Assay Description
Activation of human farnesoid X receptor in FRET assay |
J Med Chem 48: 5383-403 (2005)
Article DOI: 10.1021/jm0582221
BindingDB Entry DOI: 10.7270/Q2N29XQ6 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21724
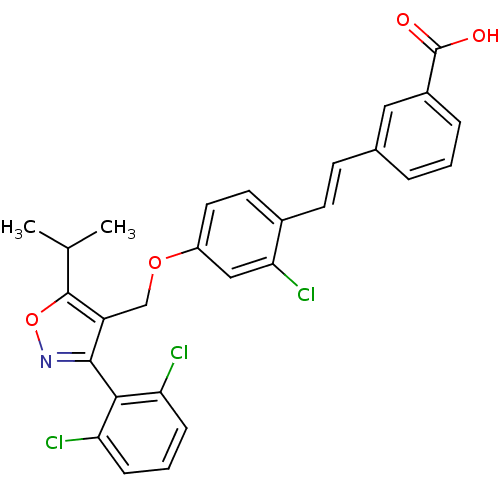
(3-[(E)-2-(2-chloro-4-{[3-(2,6-dichlorophenyl)-5-(1...)Show SMILES CC(C)c1onc(c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |(-5,9.13,;-4.28,7.76,;-2.74,7.7,;-5.11,6.46,;-6.65,6.36,;-7.03,4.87,;-5.73,4.05,;-4.54,5.03,;-3.21,4.26,;-1.87,5.03,;-.54,4.26,;.79,5.03,;2.13,4.26,;2.13,2.72,;3.46,1.95,;4.79,2.72,;6.13,1.95,;6.13,.41,;7.46,-.36,;8.79,.41,;8.79,1.95,;7.46,2.72,;10.13,2.72,;11.46,1.95,;10.13,4.26,;.79,1.95,;.79,.41,;-.54,2.72,;-5.63,2.51,;-4.28,1.78,;-2.97,2.59,;-4.23,.24,;-5.54,-.57,;-6.9,.16,;-6.94,1.7,;-8.3,2.43,)| Show InChI InChI=1S/C28H22Cl3NO4/c1-16(2)27-21(26(32-36-27)25-22(29)7-4-8-23(25)30)15-35-20-12-11-18(24(31)14-20)10-9-17-5-3-6-19(13-17)28(33)34/h3-14,16H,15H2,1-2H3,(H,33,34)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Universit£ di Perugia
Curated by ChEMBL
| Assay Description
Activation of human farnesoid X receptor |
J Med Chem 48: 5383-403 (2005)
Article DOI: 10.1021/jm0582221
BindingDB Entry DOI: 10.7270/Q2N29XQ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21674

((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihyd...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20-,22+,23+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a |
Universit£ di Perugia
Curated by ChEMBL
| Assay Description
Activation of human farnesoid X receptor; range is 10-30 |
J Med Chem 48: 5383-403 (2005)
Article DOI: 10.1021/jm0582221
BindingDB Entry DOI: 10.7270/Q2N29XQ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50171846

((E)-3-{3-[(4-Benzo[1,3]dioxol-5-yl-benzyl)-cyclohe...)Show SMILES OC(=O)\C=C\c1cccc(c1)N(Cc1ccc(cc1)-c1ccc2OCOc2c1)C(=O)C1CCCCC1 Show InChI InChI=1S/C30H29NO5/c32-29(33)16-11-21-5-4-8-26(17-21)31(30(34)24-6-2-1-3-7-24)19-22-9-12-23(13-10-22)25-14-15-27-28(18-25)36-20-35-27/h4-5,8-18,24H,1-3,6-7,19-20H2,(H,32,33)/b16-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.22E+5 | n/a | n/a | n/a | n/a |
Universit£ di Perugia
Curated by ChEMBL
| Assay Description
Activation of human farnesoid X receptor in FRET assay |
J Med Chem 48: 5383-403 (2005)
Article DOI: 10.1021/jm0582221
BindingDB Entry DOI: 10.7270/Q2N29XQ6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data