

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50084717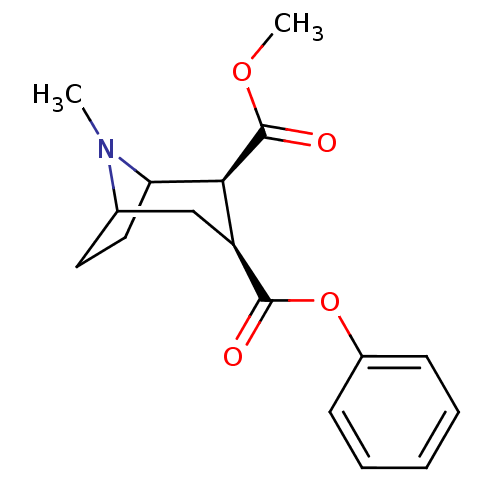 ((+)-(1R,2R,3S,5S)-methyl 3-(benzoyloxy)-8-methyl-8...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of [3H]WIN-35428 binding to rat DAT expressed in D8 cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50190380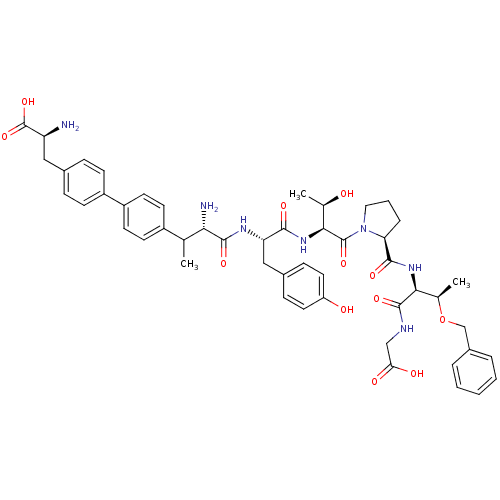 (CHEMBL374492 | bip-tyr-thr-pro-thr(obzl)-gly) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat DAT-mediated [3H]dopamine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50084717 ((+)-(1R,2R,3S,5S)-methyl 3-(benzoyloxy)-8-methyl-8...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat DAT-mediated [3H]dopamine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50084717 ((+)-(1R,2R,3S,5S)-methyl 3-(benzoyloxy)-8-methyl-8...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat SERT-mediated serotonin uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50190380 (CHEMBL374492 | bip-tyr-thr-pro-thr(obzl)-gly) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 494 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of [3H]WIN-35428 binding to rat DAT expressed in D8 cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50190379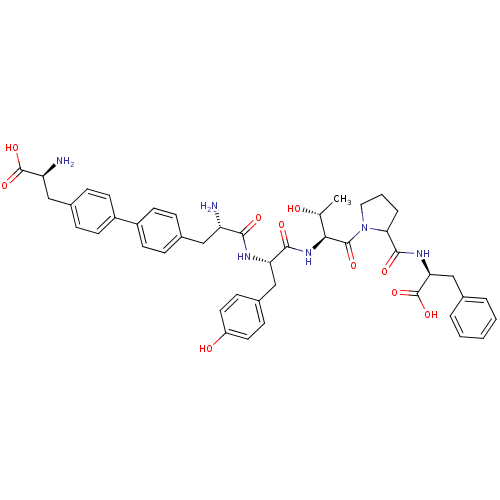 (CHEMBL439332 | bip-tyr-thr-ala-pro-phe) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat DAT-mediated [3H]dopamine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50084717 ((+)-(1R,2R,3S,5S)-methyl 3-(benzoyloxy)-8-methyl-8...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat NET-mediated norepinephrine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50190385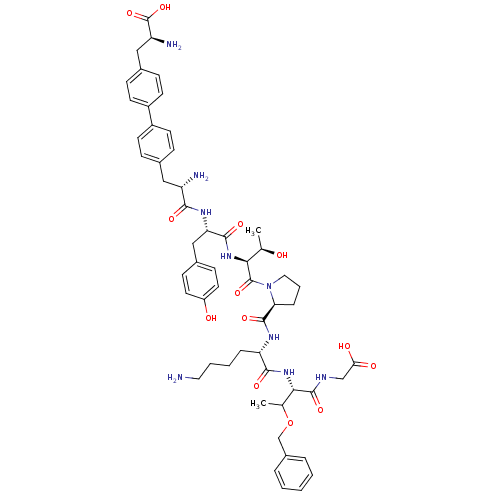 (CHEMBL219358 | bip-tyr-thr-pro-ala-thr(obzl)-gly) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat DAT-mediated [3H]dopamine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50190387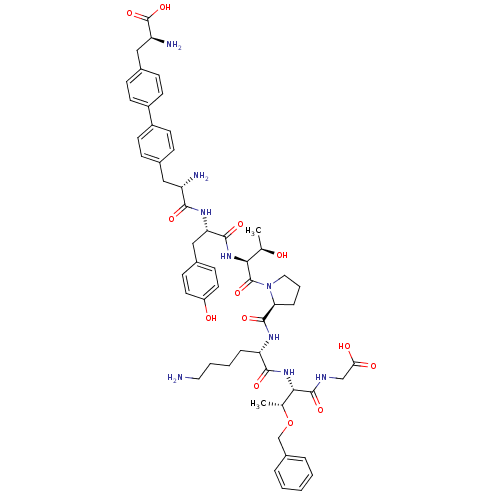 (CHEMBL428483 | bip-tyr-thr-pro-lys-thr(obzl)-gly) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat DAT-mediated [3H]dopamine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50190388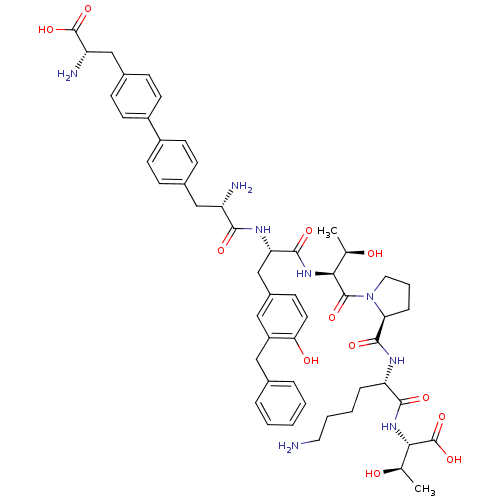 (CHEMBL385983 | bip-tyr(3bzl)-thr-pro-lys-thr) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat DAT-mediated [3H]dopamine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50190389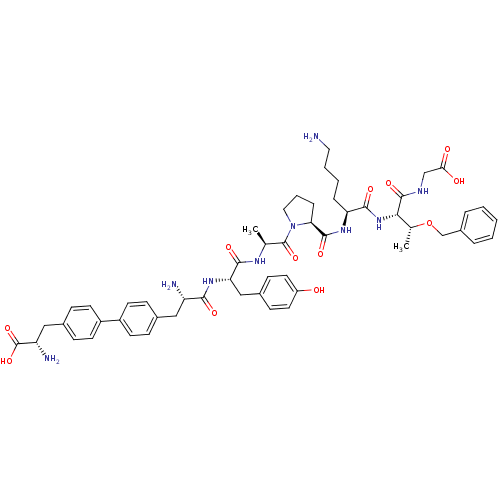 (CHEMBL277108 | bip-tyr-ala-pro-lys-thr(obzl)-gly) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat DAT-mediated [3H]dopamine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50190382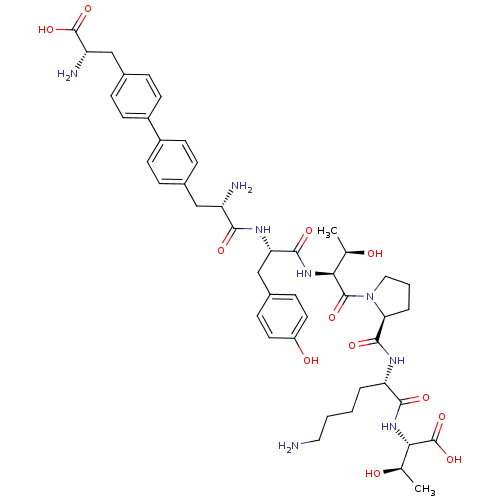 (CHEMBL379496 | bip-tyr-thr-pro-lys-thr) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat DAT-mediated [3H]dopamine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50190375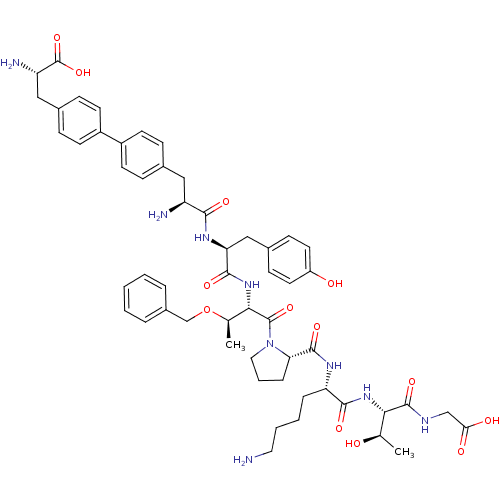 (CHEMBL269579 | bip-tyr-thr(obzl)-pro-lys-thr-gly) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat DAT-mediated [3H]dopamine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50190379 (CHEMBL439332 | bip-tyr-thr-ala-pro-phe) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat NET-mediated norepinephrine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50190379 (CHEMBL439332 | bip-tyr-thr-ala-pro-phe) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat SERT-mediated serotonin uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50190380 (CHEMBL374492 | bip-tyr-thr-pro-thr(obzl)-gly) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat NET-mediated norepinephrine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50190380 (CHEMBL374492 | bip-tyr-thr-pro-thr(obzl)-gly) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat SERT-mediated serotonin uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50190376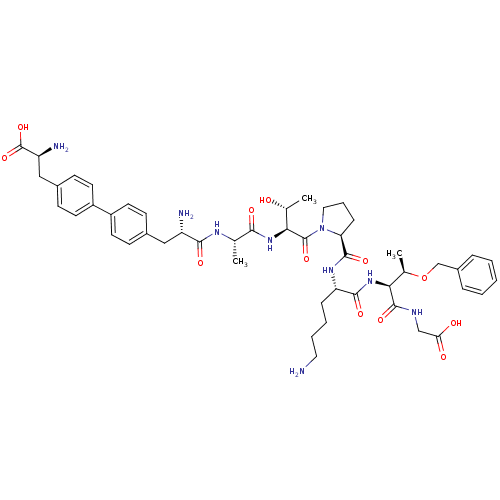 (CHEMBL383931 | bip-ala-thr-pro-lys-thr(obzl)-gly) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.73E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat DAT-mediated [3H]dopamine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50190383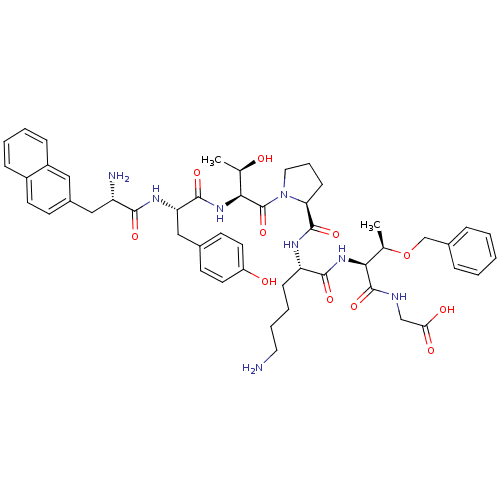 (CHEMBL411570 | nal2-tyr-thr-pro-lys-thr(obzl)-gly) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat DAT-mediated [3H]dopamine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50190384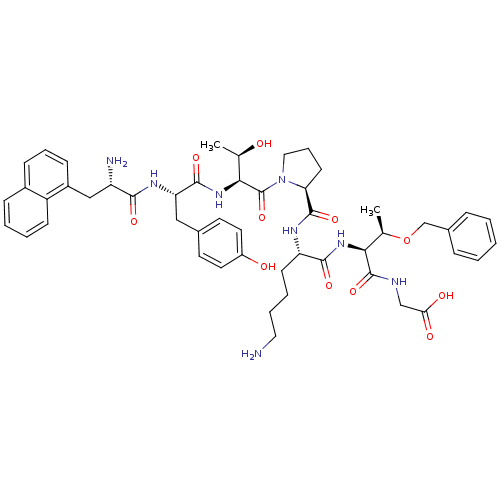 (CHEMBL218952 | nal1-tyr-thr-pro-lys-thr(obzl)-gly) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat DAT-mediated [3H]dopamine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50190386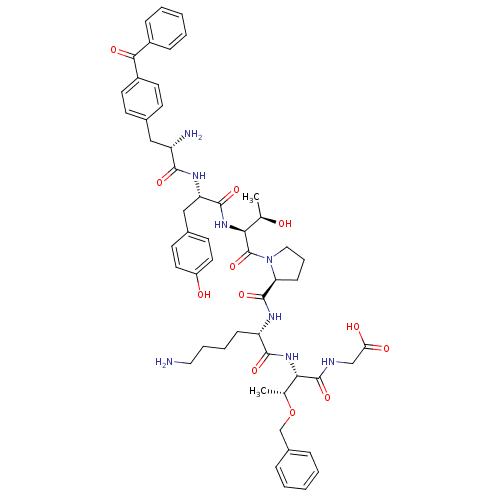 (CHEMBL409615 | bpa-tyr-thr-pro-lys-thr(obzl)-gly) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.39E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat DAT-mediated [3H]dopamine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50190382 (CHEMBL379496 | bip-tyr-thr-pro-lys-thr) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat SERT-mediated serotonin uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50190381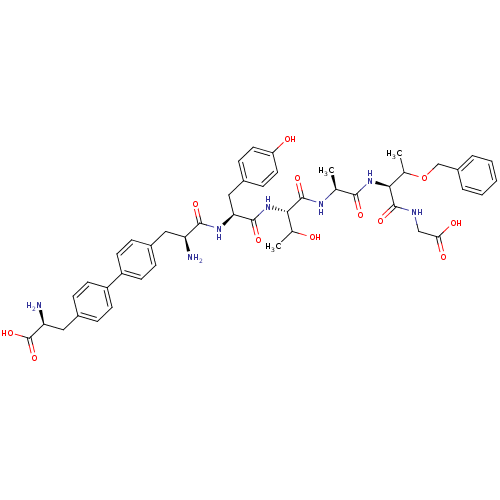 (CHEMBL209752 | bip-tyr-thr-ala-thr(obzl)-gly) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat DAT-mediated [3H]dopamine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50190377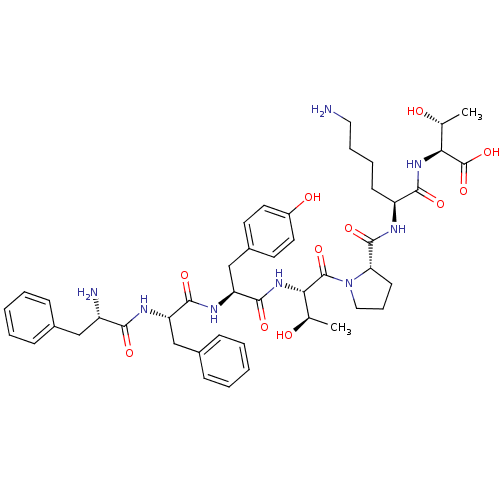 (CHEMBL212513 | phe-phe-tyr-thr-pro-lys-thr) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat DAT-mediated [3H]dopamine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50190382 (CHEMBL379496 | bip-tyr-thr-pro-lys-thr) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat NET-mediated norepinephrine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Mus musculus) | BDBM50190382 (CHEMBL379496 | bip-tyr-thr-pro-lys-thr) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse GAT1-mediated gamma-aminobutyric acid uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50190378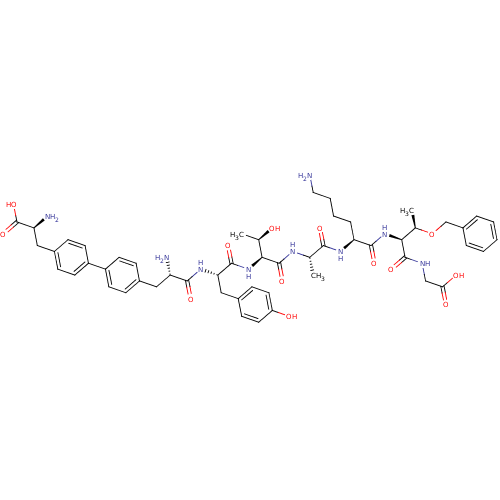 (CHEMBL219062 | bip-tyr-thr-ala-lys-thr(obzl)-gly) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat DAT-mediated [3H]dopamine uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Mus musculus) | BDBM50190379 (CHEMBL439332 | bip-tyr-thr-ala-pro-phe) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse GAT1-mediated gamma-aminobutyric acid uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent GABA transporter 1 (Mus musculus) | BDBM50190380 (CHEMBL374492 | bip-tyr-thr-pro-thr(obzl)-gly) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse GAT1-mediated gamma-aminobutyric acid uptake in CHO cells | J Med Chem 49: 4048-51 (2006) Article DOI: 10.1021/jm0601654 BindingDB Entry DOI: 10.7270/Q2FJ2HM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||