

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM29018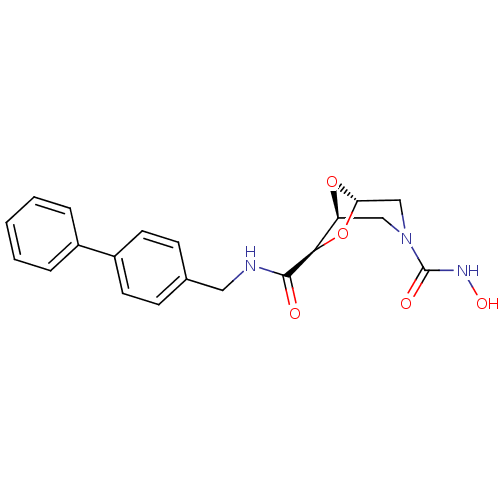 ((1S,5S,7R)-3-N-hydroxy-7-N-[(4-phenylphenyl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 1.49E+5 | 1.54E+5 | n/a | n/a | n/a | 7.0 | 25 |
University of Florence | Assay Description The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu... | Bioorg Med Chem 14: 7392-403 (2006) Article DOI: 10.1016/j.bmc.2006.07.028 BindingDB Entry DOI: 10.7270/Q2TD9VPX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM29018 ((1S,5S,7R)-3-N-hydroxy-7-N-[(4-phenylphenyl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 1.49E+5 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Florence | Assay Description The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ... | Bioorg Med Chem 14: 7392-403 (2006) Article DOI: 10.1016/j.bmc.2006.07.028 BindingDB Entry DOI: 10.7270/Q2TD9VPX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM29017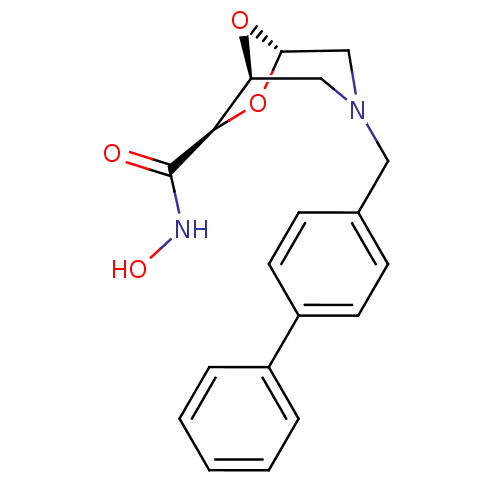 ((1S,5S,7R)-N-hydroxy-3-[(4-phenylphenyl)methyl]-6,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.99E+5 | >5.00E+5 | n/a | n/a | n/a | 7.0 | 25 |
University of Florence | Assay Description The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu... | Bioorg Med Chem 14: 7392-403 (2006) Article DOI: 10.1016/j.bmc.2006.07.028 BindingDB Entry DOI: 10.7270/Q2TD9VPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM29016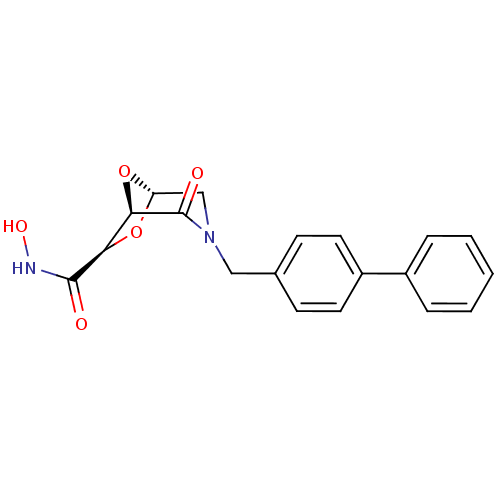 ((1R,5S,7R)-N-hydroxy-2-oxo-3-[(4-phenylphenyl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.25E+5 | >5.00E+5 | n/a | n/a | n/a | 7.0 | 25 |
University of Florence | Assay Description The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu... | Bioorg Med Chem 14: 7392-403 (2006) Article DOI: 10.1016/j.bmc.2006.07.028 BindingDB Entry DOI: 10.7270/Q2TD9VPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM29018 ((1S,5S,7R)-3-N-hydroxy-7-N-[(4-phenylphenyl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 5.13E+5 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Florence | Assay Description The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ... | Bioorg Med Chem 14: 7392-403 (2006) Article DOI: 10.1016/j.bmc.2006.07.028 BindingDB Entry DOI: 10.7270/Q2TD9VPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM29018 ((1S,5S,7R)-3-N-hydroxy-7-N-[(4-phenylphenyl)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 7.78E+5 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Florence | Assay Description The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ... | Bioorg Med Chem 14: 7392-403 (2006) Article DOI: 10.1016/j.bmc.2006.07.028 BindingDB Entry DOI: 10.7270/Q2TD9VPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM29015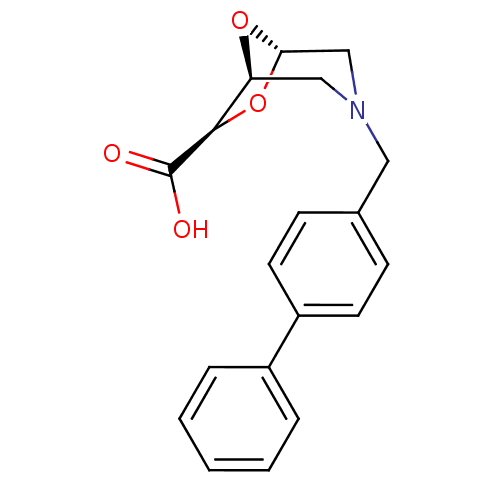 ((1S,5S,7R)-3-[(4-phenylphenyl)methyl]-6,8-dioxa-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.35E+5 | >1.00E+6 | n/a | n/a | n/a | 7.0 | 25 |
University of Florence | Assay Description The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu... | Bioorg Med Chem 14: 7392-403 (2006) Article DOI: 10.1016/j.bmc.2006.07.028 BindingDB Entry DOI: 10.7270/Q2TD9VPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM29018 ((1S,5S,7R)-3-N-hydroxy-7-N-[(4-phenylphenyl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8.65E+5 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Florence | Assay Description The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ... | Bioorg Med Chem 14: 7392-403 (2006) Article DOI: 10.1016/j.bmc.2006.07.028 BindingDB Entry DOI: 10.7270/Q2TD9VPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM29014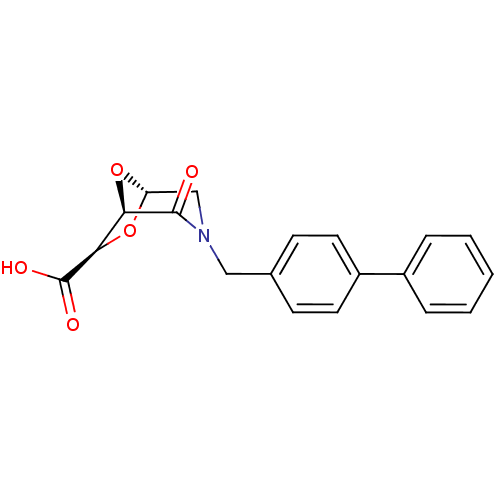 ((1R,5S,7R)-2-oxo-3-[(4-phenylphenyl)methyl]-6,8-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.54E+5 | >1.00E+6 | n/a | n/a | n/a | 7.0 | 25 |
University of Florence | Assay Description The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu... | Bioorg Med Chem 14: 7392-403 (2006) Article DOI: 10.1016/j.bmc.2006.07.028 BindingDB Entry DOI: 10.7270/Q2TD9VPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM29018 ((1S,5S,7R)-3-N-hydroxy-7-N-[(4-phenylphenyl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.18E+6 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Florence | Assay Description The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ... | Bioorg Med Chem 14: 7392-403 (2006) Article DOI: 10.1016/j.bmc.2006.07.028 BindingDB Entry DOI: 10.7270/Q2TD9VPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM29018 ((1S,5S,7R)-3-N-hydroxy-7-N-[(4-phenylphenyl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.51E+6 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Florence | Assay Description The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ... | Bioorg Med Chem 14: 7392-403 (2006) Article DOI: 10.1016/j.bmc.2006.07.028 BindingDB Entry DOI: 10.7270/Q2TD9VPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM29022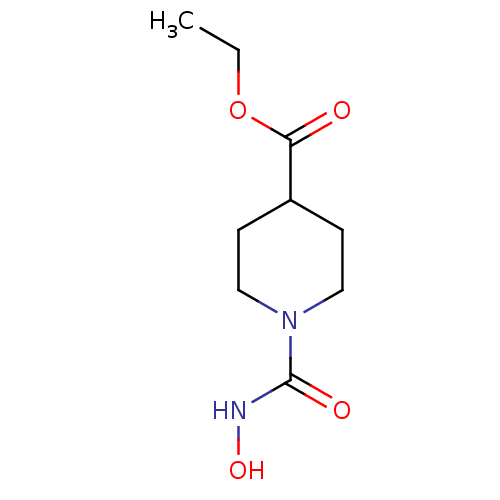 (ethyl 1-(hydroxycarbamoyl)piperidine-4-carboxylate...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 3.40E+6 | n/a | n/a | n/a | 7.2 | 25 |
University of Florence | Assay Description The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu... | Bioorg Med Chem 14: 7392-403 (2006) Article DOI: 10.1016/j.bmc.2006.07.028 BindingDB Entry DOI: 10.7270/Q2TD9VPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM29020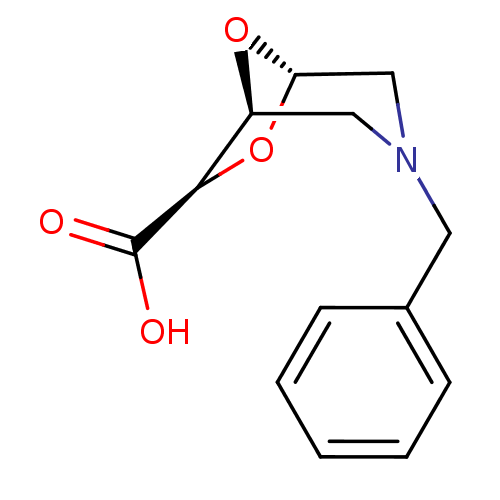 ((1S,5S,7R)-3-benzyl-6,8-dioxa-3-azabicyclo[3.2.1]o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | 7.2 | 25 |
University of Florence | Assay Description The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu... | Bioorg Med Chem 14: 7392-403 (2006) Article DOI: 10.1016/j.bmc.2006.07.028 BindingDB Entry DOI: 10.7270/Q2TD9VPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM29021 ((1R,5S,7R)-3-benzyl-N-hydroxy-2-oxo-6,8-dioxa-3-az...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | 7.2 | 25 |
University of Florence | Assay Description The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu... | Bioorg Med Chem 14: 7392-403 (2006) Article DOI: 10.1016/j.bmc.2006.07.028 BindingDB Entry DOI: 10.7270/Q2TD9VPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM29023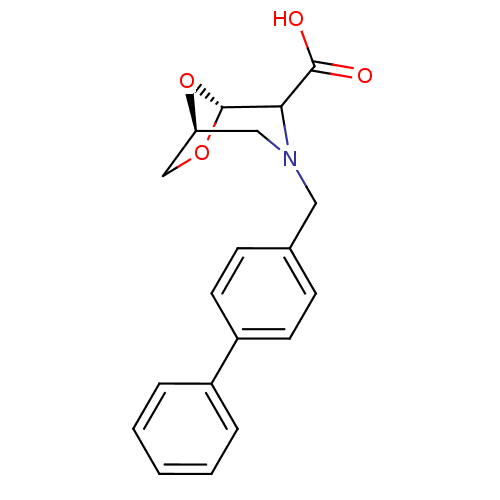 ((1S,5R)-3-[(4-phenylphenyl)methyl]-6,8-dioxa-3-aza...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
University of Florence | Assay Description The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu... | Bioorg Med Chem 14: 7392-403 (2006) Article DOI: 10.1016/j.bmc.2006.07.028 BindingDB Entry DOI: 10.7270/Q2TD9VPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM29019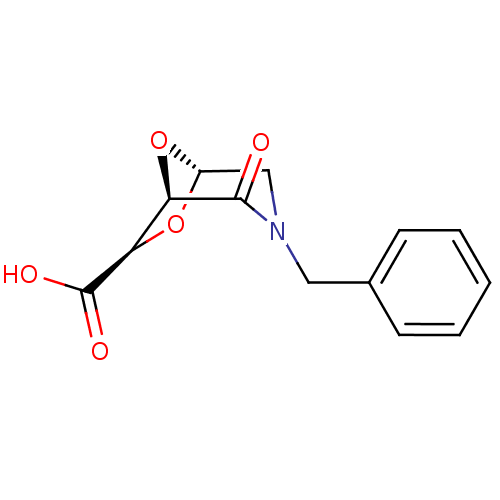 ((1R,5S,7R)-3-benzyl-2-oxo-6,8-dioxa-3-azabicyclo[3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | 7.2 | 25 |
University of Florence | Assay Description The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu... | Bioorg Med Chem 14: 7392-403 (2006) Article DOI: 10.1016/j.bmc.2006.07.028 BindingDB Entry DOI: 10.7270/Q2TD9VPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||