 Found 3 hits of Enzyme Inhibition Constant Data
Found 3 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50206984
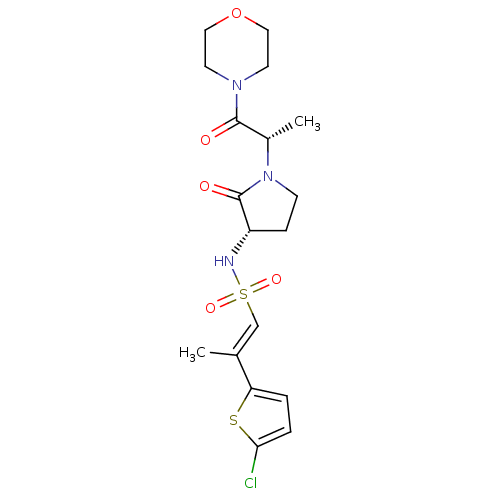
((2R)-2-(5-CHLORO-2-THIENYL)-N-{(3S)-1-[(1S)-1-METH...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)\C=C(/C)c2ccc(Cl)s2)C1=O)C(=O)N1CCOCC1 Show InChI InChI=1S/C18H24ClN3O5S2/c1-12(15-3-4-16(19)28-15)11-29(25,26)20-14-5-6-22(18(14)24)13(2)17(23)21-7-9-27-10-8-21/h3-4,11,13-14,20H,5-10H2,1-2H3/b12-11+/t13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of thrombin by fluorogenic assay |
Bioorg Med Chem Lett 17: 2931-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.034
BindingDB Entry DOI: 10.7270/Q2FJ2GFJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50206985

(2-(5-CHLORO-2-THIENYL)-N-{(3S)-1-[(1S)-1-METHYL-2-...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)CCc2ccc(Cl)s2)C1=O)C(=O)N1CCOCC1 Show InChI InChI=1S/C17H24ClN3O5S2/c1-12(16(22)20-7-9-26-10-8-20)21-6-4-14(17(21)23)19-28(24,25)11-5-13-2-3-15(18)27-13/h2-3,12,14,19H,4-11H2,1H3/t12-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of thrombin by fluorogenic assay |
Bioorg Med Chem Lett 17: 2931-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.034
BindingDB Entry DOI: 10.7270/Q2FJ2GFJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM17643
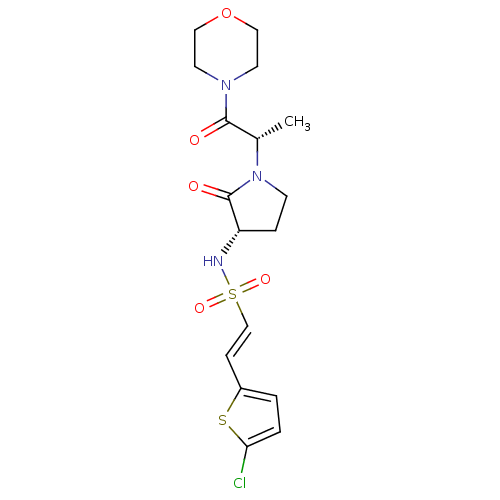
((E)-2-(5-chlorothiophen-2-yl)-N-[(3S)-1-[(2S)-1-(m...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C17H22ClN3O5S2/c1-12(16(22)20-7-9-26-10-8-20)21-6-4-14(17(21)23)19-28(24,25)11-5-13-2-3-15(18)27-13/h2-3,5,11-12,14,19H,4,6-10H2,1H3/b11-5+/t12-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 367 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of thrombin by fluorogenic assay |
Bioorg Med Chem Lett 17: 2931-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.034
BindingDB Entry DOI: 10.7270/Q2FJ2GFJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data