 Found 18 hits of Enzyme Inhibition Constant Data
Found 18 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50232748
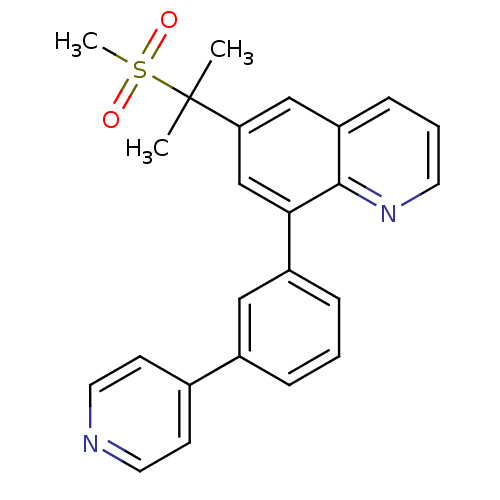
(6-(2-(methylsulfonyl)propan-2-yl)-8-(3-(pyridin-4-...)Show SMILES CC(C)(c1cc(-c2cccc(c2)-c2ccncc2)c2ncccc2c1)S(C)(=O)=O Show InChI InChI=1S/C24H22N2O2S/c1-24(2,29(3,27)28)21-15-20-8-5-11-26-23(20)22(16-21)19-7-4-6-18(14-19)17-9-12-25-13-10-17/h4-16H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50174022
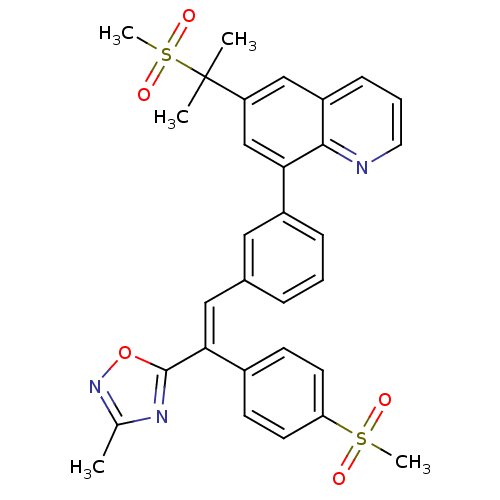
((E)-8-(3-(2-(3-methyl-1,2,4-oxadiazol-5-yl)-2-(4-(...)Show SMILES Cc1noc(n1)C(=C\c1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O)\c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H29N3O5S2/c1-20-33-30(39-34-20)28(22-11-13-26(14-12-22)40(4,35)36)17-21-8-6-9-23(16-21)27-19-25(31(2,3)41(5,37)38)18-24-10-7-15-32-29(24)27/h6-19H,1-5H3/b28-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50232743
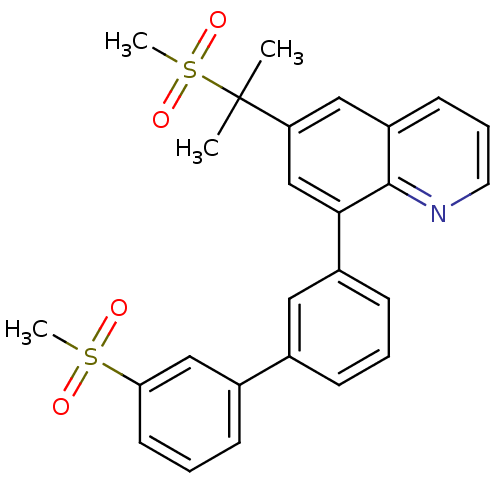
(8-(3'-methanesulfonyl-biphenyl-3-yl)-6-(1-methanes...)Show SMILES CC(C)(c1cc(-c2cccc(c2)-c2cccc(c2)S(C)(=O)=O)c2ncccc2c1)S(C)(=O)=O Show InChI InChI=1S/C26H25NO4S2/c1-26(2,33(4,30)31)22-15-21-11-7-13-27-25(21)24(17-22)20-10-5-8-18(14-20)19-9-6-12-23(16-19)32(3,28)29/h5-17H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50232730
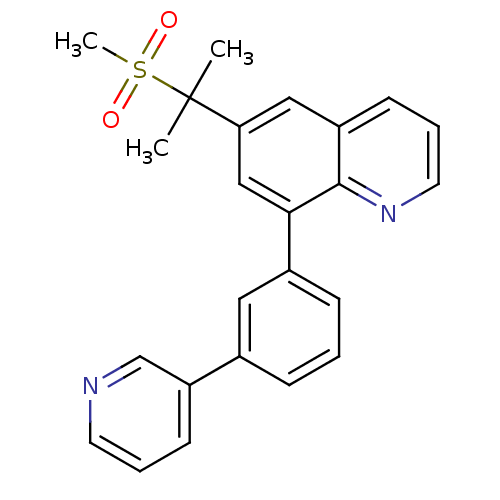
(6-(2-(methylsulfonyl)propan-2-yl)-8-(3-(pyridin-3-...)Show SMILES CC(C)(c1cc(-c2cccc(c2)-c2cccnc2)c2ncccc2c1)S(C)(=O)=O Show InChI InChI=1S/C24H22N2O2S/c1-24(2,29(3,27)28)21-14-19-9-6-12-26-23(19)22(15-21)18-8-4-7-17(13-18)20-10-5-11-25-16-20/h4-16H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50232737

(8-(4'-methanesulfonyl-biphenyl-3-yl)-6-(1-methanes...)Show SMILES CC(C)(c1cc(-c2cccc(c2)-c2ccc(cc2)S(C)(=O)=O)c2ncccc2c1)S(C)(=O)=O Show InChI InChI=1S/C26H25NO4S2/c1-26(2,33(4,30)31)22-16-21-9-6-14-27-25(21)24(17-22)20-8-5-7-19(15-20)18-10-12-23(13-11-18)32(3,28)29/h5-17H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50232733
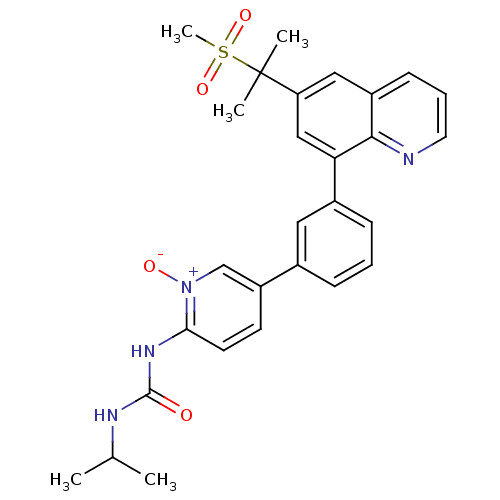
(1-isopropyl-3-(5-{3-[6-(1-methanesulfonyl-1-methyl...)Show SMILES CC(C)NC(=O)Nc1ccc(c[n+]1[O-])-c1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C28H30N4O4S/c1-18(2)30-27(33)31-25-12-11-22(17-32(25)34)19-8-6-9-20(14-19)24-16-23(28(3,4)37(5,35)36)15-21-10-7-13-29-26(21)24/h6-18H,1-5H3,(H2,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50232727
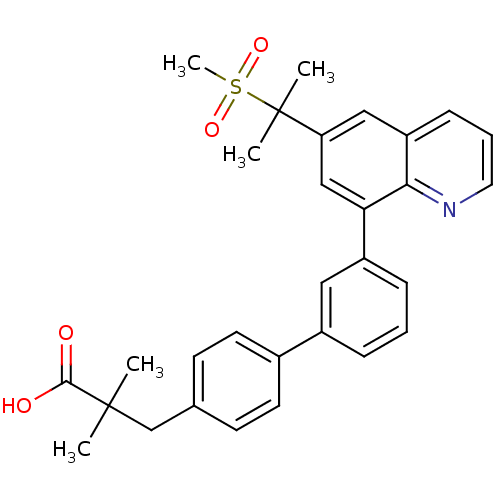
(3-{3'-[6-(1-methanesulfonyl-1-methyl-ethyl)-quinol...)Show SMILES CC(C)(Cc1ccc(cc1)-c1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O)C(O)=O Show InChI InChI=1S/C30H31NO4S/c1-29(2,28(32)33)19-20-11-13-21(14-12-20)22-8-6-9-23(16-22)26-18-25(30(3,4)36(5,34)35)17-24-10-7-15-31-27(24)26/h6-18H,19H2,1-5H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50232740
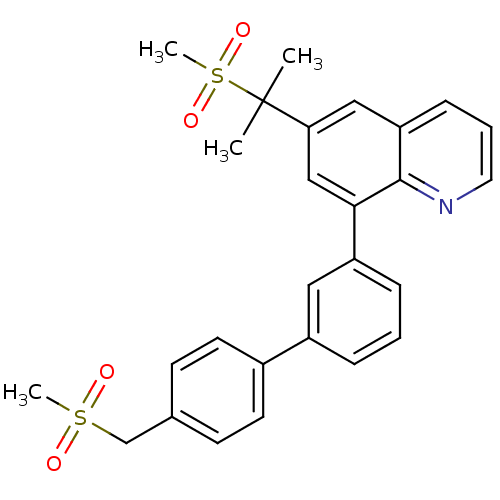
(8-(4'-methanesulfonylmethyl-biphenyl-3-yl)-6-(1-me...)Show SMILES CC(C)(c1cc(-c2cccc(c2)-c2ccc(CS(C)(=O)=O)cc2)c2ncccc2c1)S(C)(=O)=O Show InChI InChI=1S/C27H27NO4S2/c1-27(2,34(4,31)32)24-16-23-9-6-14-28-26(23)25(17-24)22-8-5-7-21(15-22)20-12-10-19(11-13-20)18-33(3,29)30/h5-17H,18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50232744

(8-(3-(1H-imidazo[4,5-b]pyridin-6-yl)phenyl)-6-(2-(...)Show SMILES CC(C)(c1cc(-c2cccc(c2)-c2cnc3nc[nH]c3c2)c2ncccc2c1)S(C)(=O)=O Show InChI InChI=1S/C25H22N4O2S/c1-25(2,32(3,30)31)20-11-18-8-5-9-26-23(18)21(13-20)17-7-4-6-16(10-17)19-12-22-24(27-14-19)29-15-28-22/h4-15H,1-3H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50232731
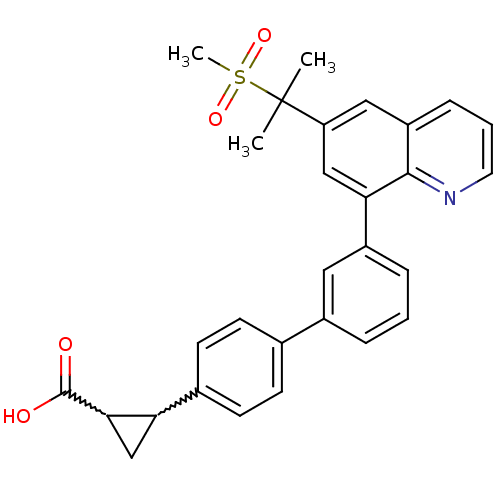
(2-{3'-[6-(1-methanesulfonyl-1-methyl-ethyl)-quinol...)Show SMILES CC(C)(c1cc(-c2cccc(c2)-c2ccc(cc2)C2CC2C(O)=O)c2ncccc2c1)S(C)(=O)=O |w:18.19,20.23| Show InChI InChI=1S/C29H27NO4S/c1-29(2,35(3,33)34)23-15-22-8-5-13-30-27(22)25(16-23)21-7-4-6-20(14-21)18-9-11-19(12-10-18)24-17-26(24)28(31)32/h4-16,24,26H,17H2,1-3H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50232731

(2-{3'-[6-(1-methanesulfonyl-1-methyl-ethyl)-quinol...)Show SMILES CC(C)(c1cc(-c2cccc(c2)-c2ccc(cc2)C2CC2C(O)=O)c2ncccc2c1)S(C)(=O)=O |w:18.19,20.23| Show InChI InChI=1S/C29H27NO4S/c1-29(2,35(3,33)34)23-15-22-8-5-13-30-27(22)25(16-23)21-7-4-6-20(14-21)18-9-11-19(12-10-18)24-17-26(24)28(31)32/h4-16,24,26H,17H2,1-3H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50232729

(5-{3-[6-(1-methanesulfonyl-1-methyl-ethyl)-quinoli...)Show SMILES CC(C)(c1cc(-c2cccc(c2)-c2ccc(C(=O)NC3CC3)[n+]([O-])c2)c2ncccc2c1)S(C)(=O)=O Show InChI InChI=1S/C28H27N3O4S/c1-28(2,36(3,34)35)22-15-20-8-5-13-29-26(20)24(16-22)19-7-4-6-18(14-19)21-9-12-25(31(33)17-21)27(32)30-23-10-11-23/h4-9,12-17,23H,10-11H2,1-3H3,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50232747
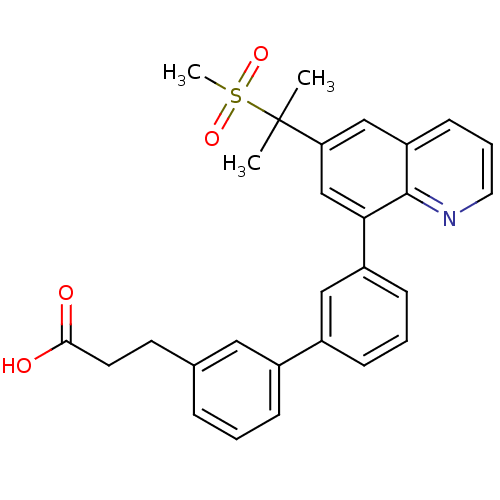
(3-{3'-[6-(1-methanesulfonyl-1-methyl-ethyl)-quinol...)Show SMILES CC(C)(c1cc(-c2cccc(c2)-c2cccc(CCC(O)=O)c2)c2ncccc2c1)S(C)(=O)=O Show InChI InChI=1S/C28H27NO4S/c1-28(2,34(3,32)33)24-17-23-11-6-14-29-27(23)25(18-24)22-10-5-9-21(16-22)20-8-4-7-19(15-20)12-13-26(30)31/h4-11,14-18H,12-13H2,1-3H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50232732
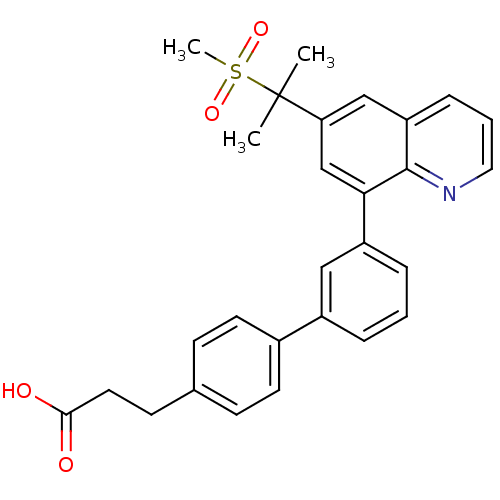
(3-{3'-[6-(1-methanesulfonyl-1-methyl-ethyl)-quinol...)Show SMILES CC(C)(c1cc(-c2cccc(c2)-c2ccc(CCC(O)=O)cc2)c2ncccc2c1)S(C)(=O)=O Show InChI InChI=1S/C28H27NO4S/c1-28(2,34(3,32)33)24-17-23-8-5-15-29-27(23)25(18-24)22-7-4-6-21(16-22)20-12-9-19(10-13-20)11-14-26(30)31/h4-10,12-13,15-18H,11,14H2,1-3H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50232752
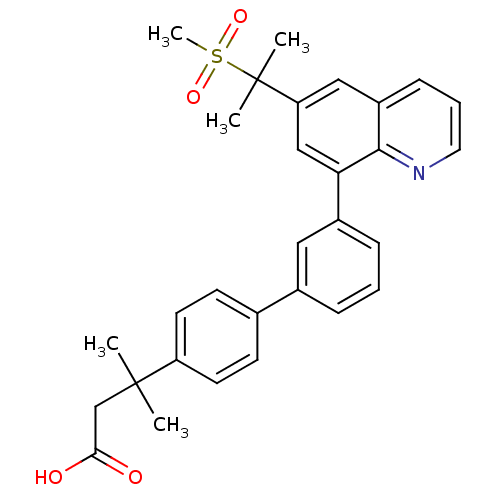
(3-{3'-[6-(1-methanesulfonyl-1-methyl-ethyl)-quinol...)Show SMILES CC(C)(CC(O)=O)c1ccc(cc1)-c1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C30H31NO4S/c1-29(2,19-27(32)33)24-13-11-20(12-14-24)21-8-6-9-22(16-21)26-18-25(30(3,4)36(5,34)35)17-23-10-7-15-31-28(23)26/h6-18H,19H2,1-5H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50232741
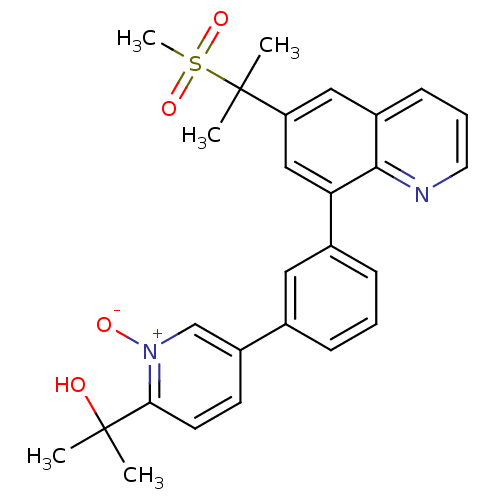
(2-(5-{3-[6-(1-methanesulfonyl-1-methyl-ethyl)-quin...)Show SMILES CC(C)(O)c1ccc(c[n+]1[O-])-c1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C27H28N2O4S/c1-26(2,30)24-12-11-21(17-29(24)31)18-8-6-9-19(14-18)23-16-22(27(3,4)34(5,32)33)15-20-10-7-13-28-25(20)23/h6-17,30H,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50232745

(6-(1-methanesulfonyl-1-methyl-ethyl)-8-[3-(6-methy...)Show SMILES Cc1ccc(c[n+]1[O-])-c1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C25H24N2O3S/c1-17-10-11-21(16-27(17)28)18-7-5-8-19(13-18)23-15-22(25(2,3)31(4,29)30)14-20-9-6-12-26-24(20)23/h5-16H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50232736
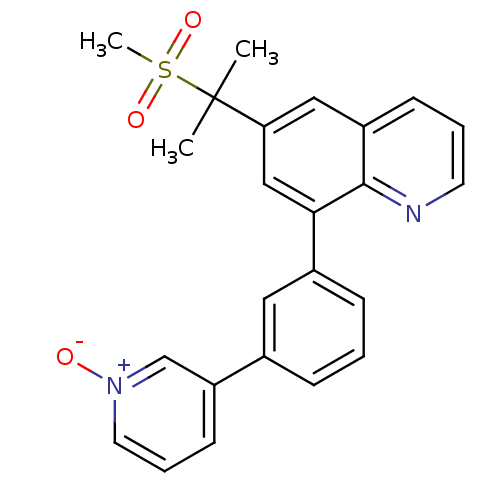
(6-(1-methanesulfonyl-1-methyl-ethyl)-8-[3-(1-oxy-p...)Show SMILES CC(C)(c1cc(-c2cccc(c2)-c2ccc[n+]([O-])c2)c2ncccc2c1)S(C)(=O)=O Show InChI InChI=1S/C24H22N2O3S/c1-24(2,30(3,28)29)21-14-19-9-5-11-25-23(19)22(15-21)18-8-4-7-17(13-18)20-10-6-12-26(27)16-20/h4-16H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1407-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.004
BindingDB Entry DOI: 10.7270/Q2VH5NKX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data