

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM86107 (Galanthamine, 7) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description Butyrylcholinesterase activity-inhibiting activities were measured by a slightly modified spectrophotometric method developed by Ellman. DTNB was us... | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM86103 (3'-Hydroxyepiglucoisatisin, 3) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description Butyrylcholinesterase activity-inhibiting activities were measured by a slightly modified spectrophotometric method developed by Ellman. DTNB was us... | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM86102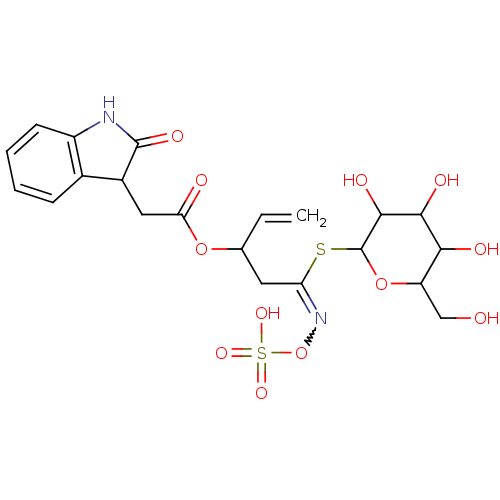 (Epiglucoisatisin, 2) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description Butyrylcholinesterase activity-inhibiting activities were measured by a slightly modified spectrophotometric method developed by Ellman. DTNB was us... | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Linoleate 9S-lipoxygenase-4 (Glycine max (Soybean) (Glycine hispida)) | BDBM50009001 (5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description LOX inhibiting activity was measured by modifying the spectrophotometric method developed by Tappel. | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM86101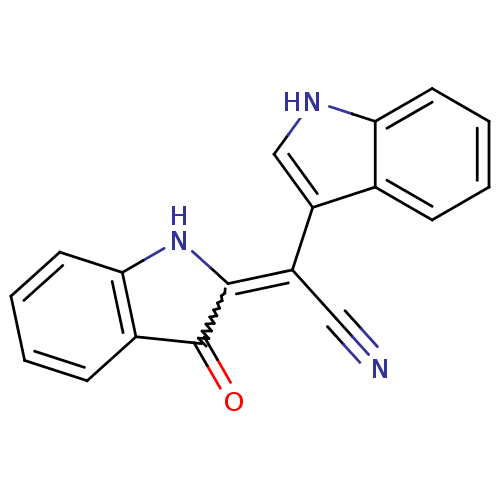 (2-[Cyano(3-indolyl) methylene]-3-indolone, 1) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description Butyrylcholinesterase activity-inhibiting activities were measured by a slightly modified spectrophotometric method developed by Ellman. DTNB was us... | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM86104 (Sulfoglucobrassicin, 4) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.48E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description Butyrylcholinesterase activity-inhibiting activities were measured by a slightly modified spectrophotometric method developed by Ellman. DTNB was us... | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Linoleate 9S-lipoxygenase-4 (Glycine max (Soybean) (Glycine hispida)) | BDBM86103 (3'-Hydroxyepiglucoisatisin, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.06E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description LOX inhibiting activity was measured by modifying the spectrophotometric method developed by Tappel. | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Linoleate 9S-lipoxygenase-4 (Glycine max (Soybean) (Glycine hispida)) | BDBM86102 (Epiglucoisatisin, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.37E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description LOX inhibiting activity was measured by modifying the spectrophotometric method developed by Tappel. | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Linoleate 9S-lipoxygenase-4 (Glycine max (Soybean) (Glycine hispida)) | BDBM86101 (2-[Cyano(3-indolyl) methylene]-3-indolone, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.91E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description LOX inhibiting activity was measured by modifying the spectrophotometric method developed by Tappel. | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Linoleate 9S-lipoxygenase-4 (Glycine max (Soybean) (Glycine hispida)) | BDBM86104 (Sulfoglucobrassicin, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.19E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description LOX inhibiting activity was measured by modifying the spectrophotometric method developed by Tappel. | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM86105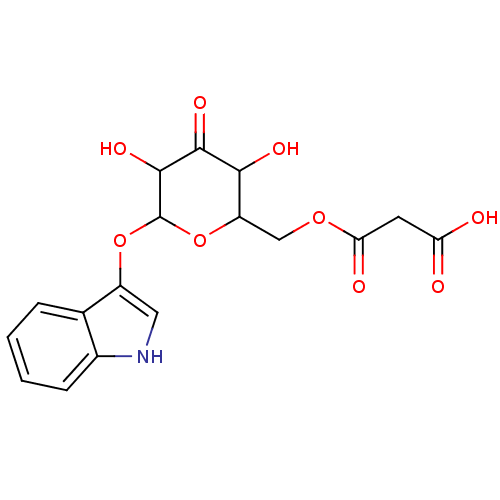 (Isatan A, 5) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.34E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description Butyrylcholinesterase activity-inhibiting activities were measured by a slightly modified spectrophotometric method developed by Ellman. DTNB was us... | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM86106 (Isatan B, 6) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.76E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description Butyrylcholinesterase activity-inhibiting activities were measured by a slightly modified spectrophotometric method developed by Ellman. DTNB was us... | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Linoleate 9S-lipoxygenase-4 (Glycine max (Soybean) (Glycine hispida)) | BDBM86105 (Isatan A, 5) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.93E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description LOX inhibiting activity was measured by modifying the spectrophotometric method developed by Tappel. | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Linoleate 9S-lipoxygenase-4 (Glycine max (Soybean) (Glycine hispida)) | BDBM86106 (Isatan B, 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.38E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description LOX inhibiting activity was measured by modifying the spectrophotometric method developed by Tappel. | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||