

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM27881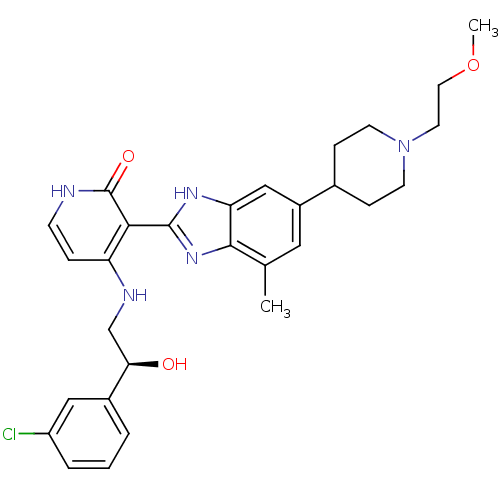 (4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM27887 (4-{[2-(4-chloro-1H-pyrazol-1-yl)ethyl]amino}-3-{6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM27884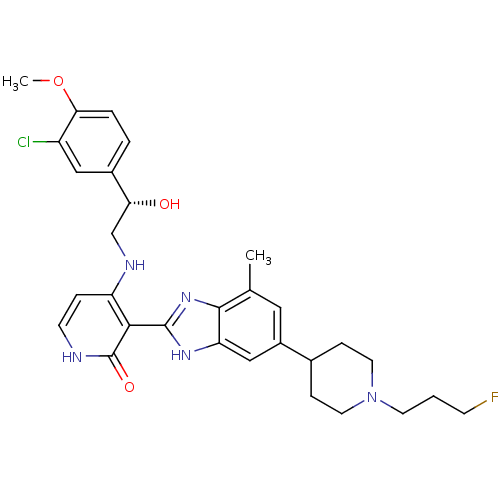 (4-{[(2S)-2-(3-chloro-4-methoxyphenyl)-2-hydroxyeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM27880 (4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM27883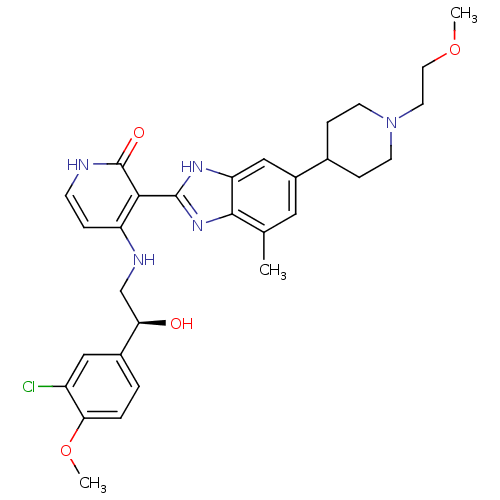 (4-{[(2S)-2-(3-chloro-4-methoxyphenyl)-2-hydroxyeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM27886 (4-{[2-(4-chloro-1H-pyrazol-1-yl)ethyl]amino}-3-{6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM27885 (4-{[2-(4-chloro-1H-pyrazol-1-yl)ethyl]amino}-3-[4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM27882 (4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM27888 (4-{[2-(4-chloro-1H-pyrazol-1-yl)ethyl]amino}-3-{6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM27879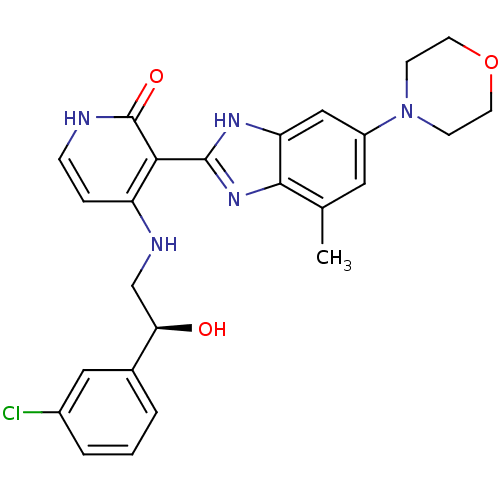 (4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company | Assay Description The inhibition of recombinant human CYP3A4 was measured as the ability to perform a dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin (BFC). Befo... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM27879 (4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM27883 (4-{[(2S)-2-(3-chloro-4-methoxyphenyl)-2-hydroxyeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company | Assay Description The inhibition of recombinant human CYP3A4 was measured as the ability to perform a dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin (BFC). Befo... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM27884 (4-{[(2S)-2-(3-chloro-4-methoxyphenyl)-2-hydroxyeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company | Assay Description The inhibition of recombinant human CYP3A4 was measured as the ability to perform a dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin (BFC). Befo... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM27881 (4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company | Assay Description The inhibition of recombinant human CYP3A4 was measured as the ability to perform a dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin (BFC). Befo... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM27880 (4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company | Assay Description The inhibition of recombinant human CYP3A4 was measured as the ability to perform a dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin (BFC). Befo... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM27887 (4-{[2-(4-chloro-1H-pyrazol-1-yl)ethyl]amino}-3-{6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company | Assay Description The inhibition of recombinant human CYP3A4 was measured as the ability to perform a dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin (BFC). Befo... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM27886 (4-{[2-(4-chloro-1H-pyrazol-1-yl)ethyl]amino}-3-{6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company | Assay Description The inhibition of recombinant human CYP3A4 was measured as the ability to perform a dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin (BFC). Befo... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM27888 (4-{[2-(4-chloro-1H-pyrazol-1-yl)ethyl]amino}-3-{6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company | Assay Description The inhibition of recombinant human CYP3A4 was measured as the ability to perform a dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin (BFC). Befo... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM27882 (4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company | Assay Description The inhibition of recombinant human CYP3A4 was measured as the ability to perform a dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin (BFC). Befo... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM27885 (4-{[2-(4-chloro-1H-pyrazol-1-yl)ethyl]amino}-3-[4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company | Assay Description The inhibition of recombinant human CYP3A4 was measured as the ability to perform a dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin (BFC). Befo... | J Med Chem 51: 5897-900 (2008) Article DOI: 10.1021/jm800832q BindingDB Entry DOI: 10.7270/Q2513WJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||