

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50003187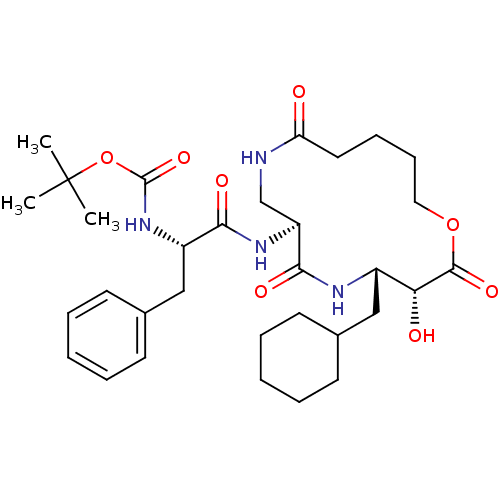 (CHEMBL92538 | [1-(4-Cyclohexylmethyl-3-hydroxy-2,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description The compound was evaluated in vitro for inhibition of human plasma renin at pH 7.4. | J Med Chem 34: 2692-701 (1991) BindingDB Entry DOI: 10.7270/Q2P26X4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009620 (CHEMBL431854 | [1-(11-Cyclohexylmethyl-12-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description The compound was evaluated in vitro for inhibition of human plasma renin at pH 7.4. | J Med Chem 34: 2692-701 (1991) BindingDB Entry DOI: 10.7270/Q2P26X4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009621 (CHEMBL90266 | [1-(13-Cyclohexylmethyl-11,12-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description The compound was evaluated in vitro for inhibition of human plasma renin at pH 7.4. | J Med Chem 34: 2692-701 (1991) BindingDB Entry DOI: 10.7270/Q2P26X4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50003186 (CHEMBL327710 | [1-(4-Cyclohexylmethyl-3-hydroxy-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description The compound was evaluated in vitro for inhibition of human plasma renin at pH 7.4. | J Med Chem 34: 2692-701 (1991) BindingDB Entry DOI: 10.7270/Q2P26X4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009622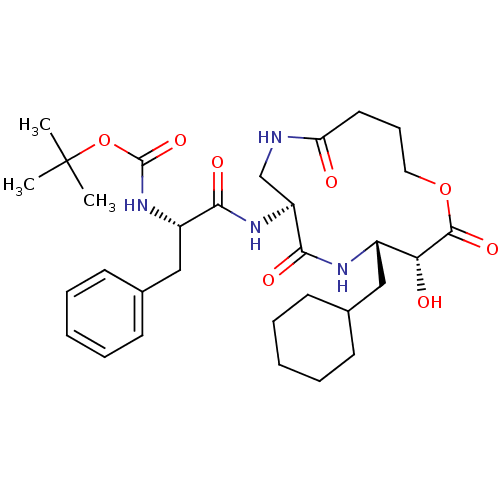 (CHEMBL327709 | [1-(4-Cyclohexylmethyl-3-hydroxy-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description The compound was evaluated in vitro for inhibition of human plasma renin at pH 7.4. | J Med Chem 34: 2692-701 (1991) BindingDB Entry DOI: 10.7270/Q2P26X4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009619 (CHEMBL91847 | [1-(13-Cyclohexylmethyl-12-hydroxy-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description The compound was evaluated in vitro for inhibition of human plasma renin at pH 7.4. | J Med Chem 34: 2692-701 (1991) BindingDB Entry DOI: 10.7270/Q2P26X4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009623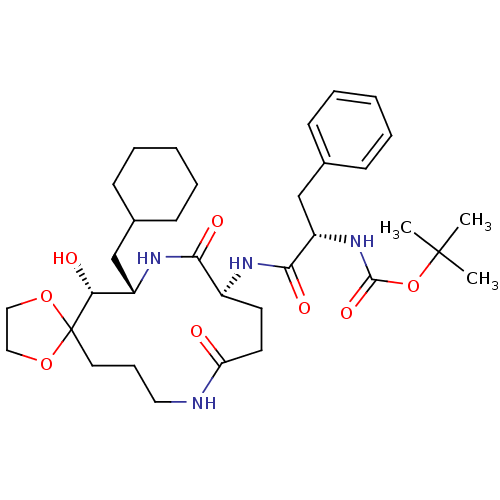 (CHEMBL330455 | [1-(7-Cyclohexylmethyl-6-hydroxy-9,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description The compound was evaluated in vitro for inhibition of human plasma renin at pH 7.4. | J Med Chem 34: 2692-701 (1991) BindingDB Entry DOI: 10.7270/Q2P26X4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50009624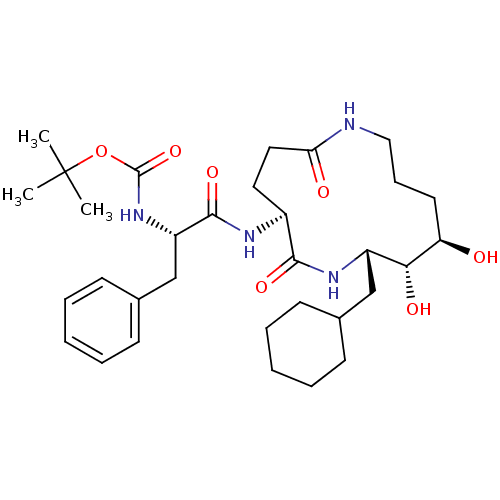 (CHEMBL327811 | [1-(13-Cyclohexylmethyl-11,12-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description The compound was evaluated in vitro for inhibition of human plasma renin at pH 7.4. | J Med Chem 34: 2692-701 (1991) BindingDB Entry DOI: 10.7270/Q2P26X4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||