

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50246062 (2-(4-phenoxyphenoxy)ethanamine | CHEMBL472386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of epoxide hydrolase activity of human leukotriene A4 hydrolase assessed as LTB4 level by ELISA | J Med Chem 51: 7882-8 (2008) Article DOI: 10.1021/jm8010096 BindingDB Entry DOI: 10.7270/Q2VH5NQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50246062 (2-(4-phenoxyphenoxy)ethanamine | CHEMBL472386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of aminopeptidase activity of human leukotriene A4 hydrolase assessed as para-nitroanilide release by spectrophotometry | J Med Chem 51: 7882-8 (2008) Article DOI: 10.1021/jm8010096 BindingDB Entry DOI: 10.7270/Q2VH5NQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50246062 (2-(4-phenoxyphenoxy)ethanamine | CHEMBL472386) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 | J Med Chem 51: 7882-8 (2008) Article DOI: 10.1021/jm8010096 BindingDB Entry DOI: 10.7270/Q2VH5NQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50247069 (2-(7-(benzyloxy)-1H-indol-3-yl)ethanamine | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of aminopeptidase activity of human leukotriene A4 hydrolase assessed as para-nitroanilide release by spectrophotometry | J Med Chem 51: 7882-8 (2008) Article DOI: 10.1021/jm8010096 BindingDB Entry DOI: 10.7270/Q2VH5NQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50247033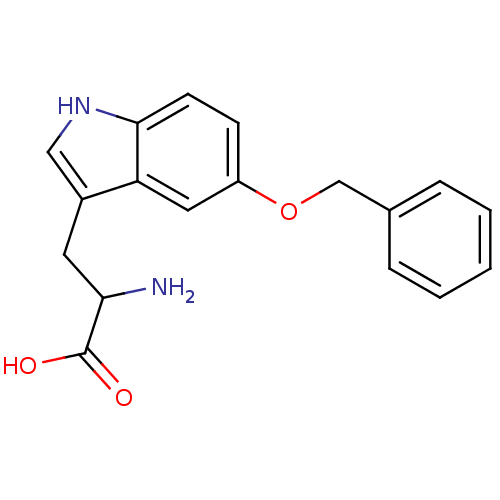 (2-amino-3-(5-(benzyloxy)-1H-indol-3-yl)propanoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of aminopeptidase activity of human leukotriene A4 hydrolase assessed as para-nitroanilide release by spectrophotometry | J Med Chem 51: 7882-8 (2008) Article DOI: 10.1021/jm8010096 BindingDB Entry DOI: 10.7270/Q2VH5NQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50247034 (4-(4-Nitro-benzyl)-1-(2-oxo-2-phenyl-ethyl)-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of aminopeptidase activity of human leukotriene A4 hydrolase assessed as para-nitroanilide release by spectrophotometry | J Med Chem 51: 7882-8 (2008) Article DOI: 10.1021/jm8010096 BindingDB Entry DOI: 10.7270/Q2VH5NQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50247033 (2-amino-3-(5-(benzyloxy)-1H-indol-3-yl)propanoic a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 | J Med Chem 51: 7882-8 (2008) Article DOI: 10.1021/jm8010096 BindingDB Entry DOI: 10.7270/Q2VH5NQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50247034 (4-(4-Nitro-benzyl)-1-(2-oxo-2-phenyl-ethyl)-pyridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 | J Med Chem 51: 7882-8 (2008) Article DOI: 10.1021/jm8010096 BindingDB Entry DOI: 10.7270/Q2VH5NQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50247069 (2-(7-(benzyloxy)-1H-indol-3-yl)ethanamine | CHEMBL...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 | J Med Chem 51: 7882-8 (2008) Article DOI: 10.1021/jm8010096 BindingDB Entry DOI: 10.7270/Q2VH5NQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50247069 (2-(7-(benzyloxy)-1H-indol-3-yl)ethanamine | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of epoxide hydrolase activity of human leukotriene A4 hydrolase assessed as LTB4 level by ELISA | J Med Chem 51: 7882-8 (2008) Article DOI: 10.1021/jm8010096 BindingDB Entry DOI: 10.7270/Q2VH5NQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50247033 (2-amino-3-(5-(benzyloxy)-1H-indol-3-yl)propanoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of epoxide hydrolase activity of human leukotriene A4 hydrolase assessed as LTB4 level by ELISA | J Med Chem 51: 7882-8 (2008) Article DOI: 10.1021/jm8010096 BindingDB Entry DOI: 10.7270/Q2VH5NQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50247034 (4-(4-Nitro-benzyl)-1-(2-oxo-2-phenyl-ethyl)-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of epoxide hydrolase activity of human leukotriene A4 hydrolase assessed as LTB4 level by ELISA | J Med Chem 51: 7882-8 (2008) Article DOI: 10.1021/jm8010096 BindingDB Entry DOI: 10.7270/Q2VH5NQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||