

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retinoic acid receptor beta (Mus musculus) | BDBM50346144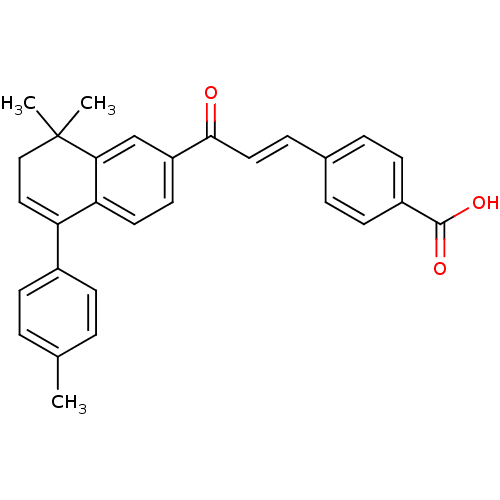 (4-[(1E)-3-(7,8-Dihydro-8,8-dimethyl-5-p-tolylnapht...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Antagonist activity at yeast GAL4 fused mouse RARbeta ligand binding domain expressed in HeLa cells assessed as inhibition of TTNPB-induced receptor ... | Bioorg Med Chem 17: 4345-59 (2009) Article DOI: 10.1016/j.bmc.2009.05.035 BindingDB Entry DOI: 10.7270/Q2P26ZF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Mus musculus) | BDBM50346143 (CHEMBL1783648 | rac-4-[(1E)-3-(7,8-Dihydro-8,8-dim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Antagonist activity at yeast GAL4 fused mouse RARalpha ligand binding domain expressed in HeLa cells assessed as inhibition of TTNPB-induced receptor... | Bioorg Med Chem 17: 4345-59 (2009) Article DOI: 10.1016/j.bmc.2009.05.035 BindingDB Entry DOI: 10.7270/Q2P26ZF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Mus musculus) | BDBM50346143 (CHEMBL1783648 | rac-4-[(1E)-3-(7,8-Dihydro-8,8-dim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Antagonist activity at yeast GAL4 fused mouse RARbeta ligand binding domain expressed in HeLa cells assessed as inhibition of TTNPB-induced receptor ... | Bioorg Med Chem 17: 4345-59 (2009) Article DOI: 10.1016/j.bmc.2009.05.035 BindingDB Entry DOI: 10.7270/Q2P26ZF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Mus musculus) | BDBM50346146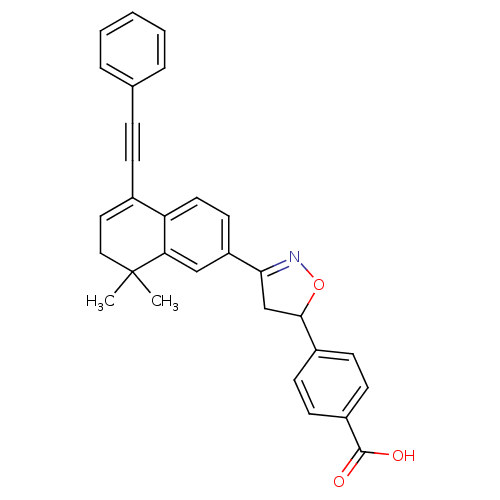 (CHEMBL1783651 | rac-4-[3-(8,8-Dimethyl-5-(phenylet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Antagonist activity at yeast GAL4 fused mouse RARgamma ligand binding domain expressed in HeLa cells assessed as inhibition of TTNPB-induced receptor... | Bioorg Med Chem 17: 4345-59 (2009) Article DOI: 10.1016/j.bmc.2009.05.035 BindingDB Entry DOI: 10.7270/Q2P26ZF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Mus musculus) | BDBM50346145 (CHEMBL1783649 | rac-4-[(3-(8,8-Dimethyl-5-phenyl-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Antagonist activity at yeast GAL4 fused mouse RARgamma ligand binding domain expressed in HeLa cells assessed as inhibition of TTNPB-induced receptor... | Bioorg Med Chem 17: 4345-59 (2009) Article DOI: 10.1016/j.bmc.2009.05.035 BindingDB Entry DOI: 10.7270/Q2P26ZF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||