 Found 124 hits of Enzyme Inhibition Constant Data
Found 124 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50416771
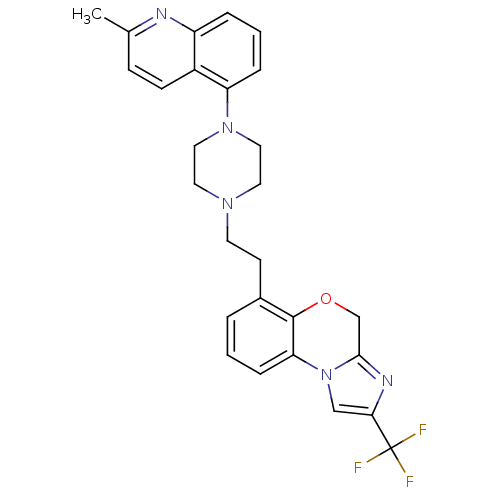
(CHEMBL1241641)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2nc(cn-32)C(F)(F)F)CC1 Show InChI InChI=1S/C27H26F3N5O/c1-18-8-9-20-21(31-18)5-3-6-22(20)34-14-12-33(13-15-34)11-10-19-4-2-7-23-26(19)36-17-25-32-24(16-35(23)25)27(28,29)30/h2-9,16H,10-15,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50412441
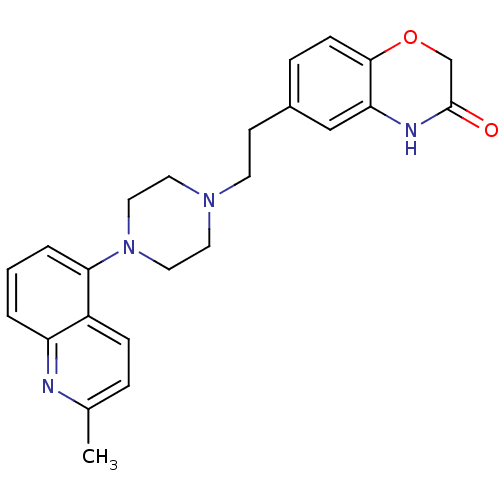
(CHEMBL490417 | SB-744185)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-5-7-19-20(25-17)3-2-4-22(19)28-13-11-27(12-14-28)10-9-18-6-8-23-21(15-18)26-24(29)16-30-23/h2-8,15H,9-14,16H2,1H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326147

(CHEMBL1242345 | N-(cyclopropylmethyl)-6-(2-(4-(2-m...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)NCC2CC2)CC1 Show InChI InChI=1S/C31H34N6O2/c1-21-8-11-24-25(34-21)5-3-6-26(24)36-16-14-35(15-17-36)13-12-23-4-2-7-27-30(23)39-19-28-29(33-20-37(27)28)31(38)32-18-22-9-10-22/h2-8,11,20,22H,9-10,12-19H2,1H3,(H,32,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50416772

(CHEMBL1242257)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)NC2CCCC2)CC1 Show InChI InChI=1S/C32H36N6O2/c1-22-12-13-25-26(34-22)9-5-10-27(25)37-18-16-36(17-19-37)15-14-23-6-4-11-28-31(23)40-20-29-30(33-21-38(28)29)32(39)35-24-7-2-3-8-24/h4-6,9-13,21,24H,2-3,7-8,14-20H2,1H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50416769
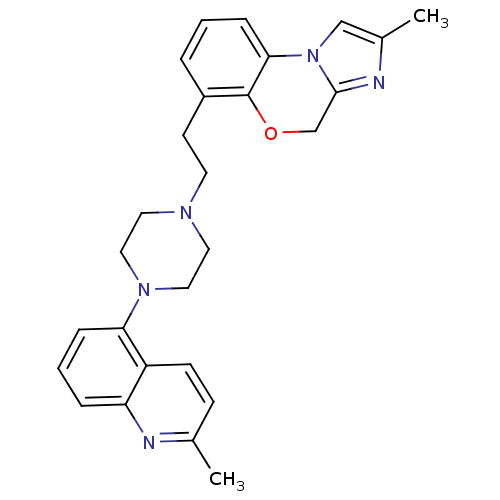
(CHEMBL1241547)Show SMILES Cc1cn-2c(COc3c(CCN4CCN(CC4)c4cccc5nc(C)ccc45)cccc-23)n1 Show InChI InChI=1S/C27H29N5O/c1-19-9-10-22-23(28-19)6-4-7-24(22)31-15-13-30(14-16-31)12-11-21-5-3-8-25-27(21)33-18-26-29-20(2)17-32(25)26/h3-10,17H,11-16,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50416770

(CHEMBL1241548)Show SMILES Cc1nc2COc3c(CCN4CCN(CC4)c4cccc5nc(C)ccc45)cccc3-n2c1C Show InChI InChI=1S/C28H31N5O/c1-19-10-11-23-24(29-19)7-5-8-25(23)32-16-14-31(15-17-32)13-12-22-6-4-9-26-28(22)34-18-27-30-20(2)21(3)33(26)27/h4-11H,12-18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326134
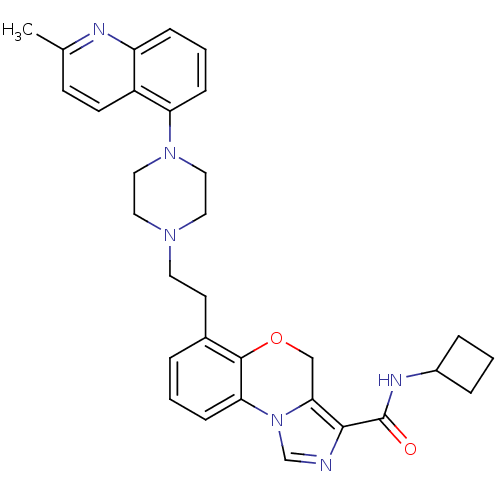
(CHEMBL1242167 | N-cyclobutyl-6-(2-(4-(2-methylquin...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)NC2CCC2)CC1 Show InChI InChI=1S/C31H34N6O2/c1-21-11-12-24-25(33-21)8-4-9-26(24)36-17-15-35(16-18-36)14-13-22-5-2-10-27-30(22)39-19-28-29(32-20-37(27)28)31(38)34-23-6-3-7-23/h2,4-5,8-12,20,23H,3,6-7,13-19H2,1H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326139
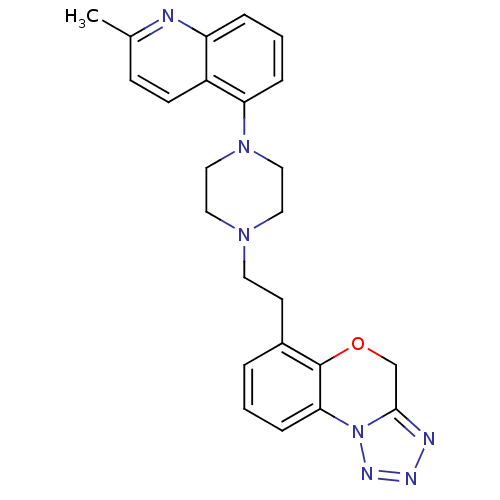
(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2nnnn-32)CC1 Show InChI InChI=1S/C24H25N7O/c1-17-8-9-19-20(25-17)5-3-6-21(19)30-14-12-29(13-15-30)11-10-18-4-2-7-22-24(18)32-16-23-26-27-28-31(22)23/h2-9H,10-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326135

(CHEMBL1242436 | azetidin-1-yl(6-(2-(4-(2-methylqui...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCC2)CC1 Show InChI InChI=1S/C30H32N6O2/c1-21-9-10-23-24(32-21)6-3-7-25(23)34-17-15-33(16-18-34)14-11-22-5-2-8-26-29(22)38-19-27-28(31-20-36(26)27)30(37)35-12-4-13-35/h2-3,5-10,20H,4,11-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326146

(CHEMBL1242081 | N-cyclopropyl-6-(2-(4-(2-methylqui...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)NC2CC2)CC1 Show InChI InChI=1S/C30H32N6O2/c1-20-8-11-23-24(32-20)5-3-6-25(23)35-16-14-34(15-17-35)13-12-21-4-2-7-26-29(21)38-18-27-28(31-19-36(26)27)30(37)33-22-9-10-22/h2-8,11,19,22H,9-10,12-18H2,1H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50413077
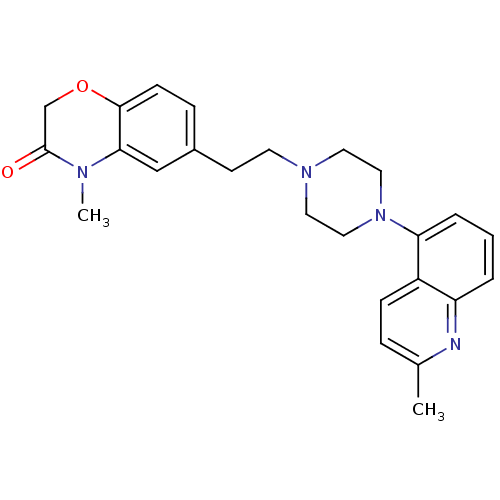
(CHEMBL522257)Show SMILES CN1C(=O)COc2ccc(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cc12 Show InChI InChI=1S/C25H28N4O2/c1-18-6-8-20-21(26-18)4-3-5-22(20)29-14-12-28(13-15-29)11-10-19-7-9-24-23(16-19)27(2)25(30)17-31-24/h3-9,16H,10-15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326140
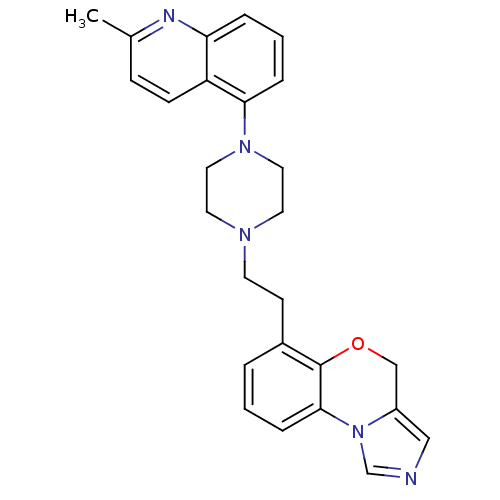
(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2cncn-32)CC1 Show InChI InChI=1S/C26H27N5O/c1-19-8-9-22-23(28-19)5-3-6-24(22)30-14-12-29(13-15-30)11-10-20-4-2-7-25-26(20)32-17-21-16-27-18-31(21)25/h2-9,16,18H,10-15,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326149
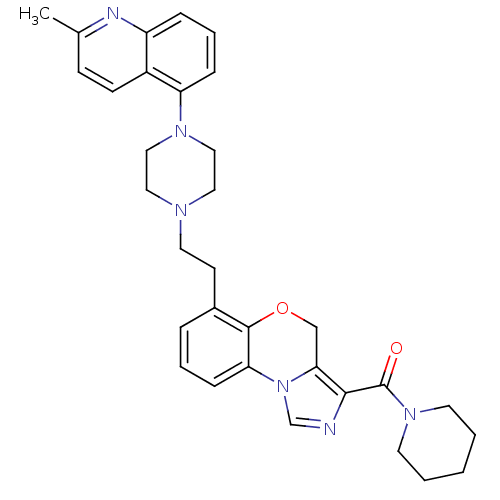
((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCCCC2)CC1 Show InChI InChI=1S/C32H36N6O2/c1-23-11-12-25-26(34-23)8-6-9-27(25)36-19-17-35(18-20-36)16-13-24-7-5-10-28-31(24)40-21-29-30(33-22-38(28)29)32(39)37-14-3-2-4-15-37/h5-12,22H,2-4,13-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326137

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C#N)CC1 Show InChI InChI=1S/C27H26N6O/c1-19-8-9-21-22(30-19)5-3-6-24(21)32-14-12-31(13-15-32)11-10-20-4-2-7-25-27(20)34-17-26-23(16-28)29-18-33(25)26/h2-9,18H,10-15,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326148
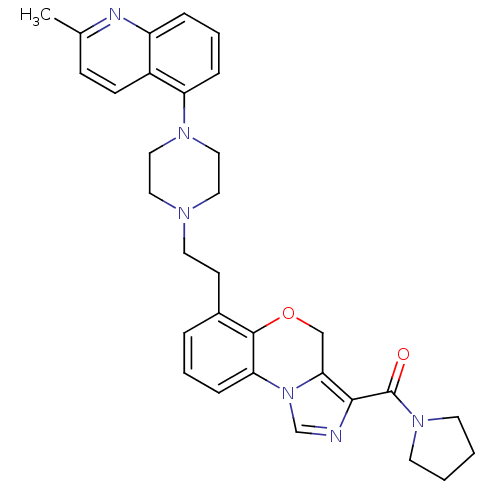
((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCCC2)CC1 Show InChI InChI=1S/C31H34N6O2/c1-22-10-11-24-25(33-22)7-5-8-26(24)35-18-16-34(17-19-35)15-12-23-6-4-9-27-30(23)39-20-28-29(32-21-37(27)28)31(38)36-13-2-3-14-36/h4-11,21H,2-3,12-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326133

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2nccn-32)CC1 Show InChI InChI=1S/C26H27N5O/c1-19-8-9-21-22(28-19)5-3-6-23(21)30-16-14-29(15-17-30)12-10-20-4-2-7-24-26(20)32-18-25-27-11-13-31(24)25/h2-9,11,13H,10,12,14-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326136

(1-(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)e...)Show SMILES CO\N=C(/C)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C29H32N6O2/c1-20-10-11-23-24(31-20)7-5-8-25(23)34-16-14-33(15-17-34)13-12-22-6-4-9-26-29(22)37-18-27-28(21(2)32-36-3)30-19-35(26)27/h4-11,19H,12-18H2,1-3H3/b32-21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50413549
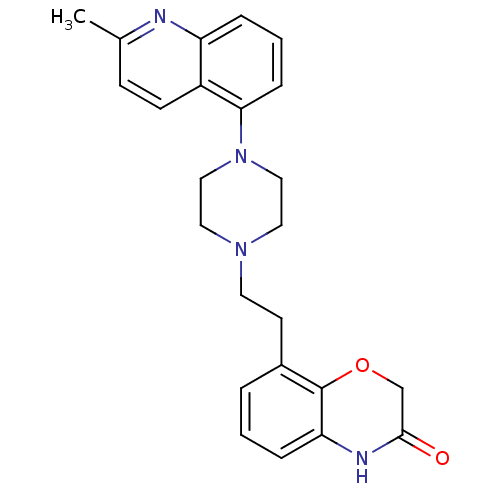
(CHEMBL513715)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3NC(=O)COc23)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-8-9-19-20(25-17)5-3-7-22(19)28-14-12-27(13-15-28)11-10-18-4-2-6-21-24(18)30-16-23(29)26-21/h2-9H,10-16H2,1H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326142

(3-methyl-6-(2-(4-(2-methylquinolin-5-yl)piperazin-...)Show SMILES Cc1nnn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C26H28N6O/c1-18-9-10-21-22(27-18)6-4-7-23(21)31-15-13-30(14-16-31)12-11-20-5-3-8-24-26(20)33-17-25-19(2)28-29-32(24)25/h3-10H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326145

(CHEMBL1241998 | N-isopropyl-6-(2-(4-(2-methylquino...)Show SMILES CC(C)NC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C30H34N6O2/c1-20(2)32-30(37)28-27-18-38-29-22(6-4-9-26(29)36(27)19-31-28)12-13-34-14-16-35(17-15-34)25-8-5-7-24-23(25)11-10-21(3)33-24/h4-11,19-20H,12-18H2,1-3H3,(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326144
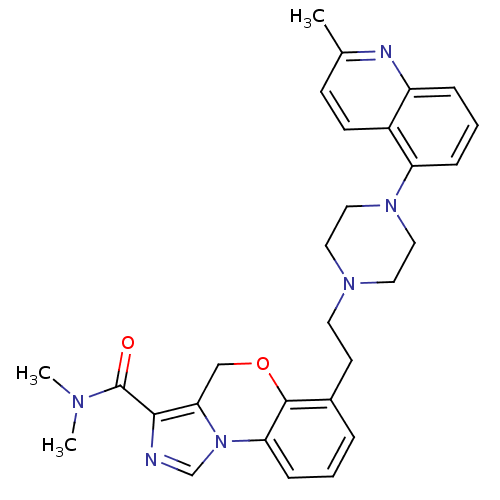
(CHEMBL1241824 | N,N-dimethyl-6-(2-(4-(2-methylquin...)Show SMILES CN(C)C(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C29H32N6O2/c1-20-10-11-22-23(31-20)7-5-8-24(22)34-16-14-33(15-17-34)13-12-21-6-4-9-25-28(21)37-18-26-27(29(36)32(2)3)30-19-35(25)26/h4-11,19H,12-18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50413550
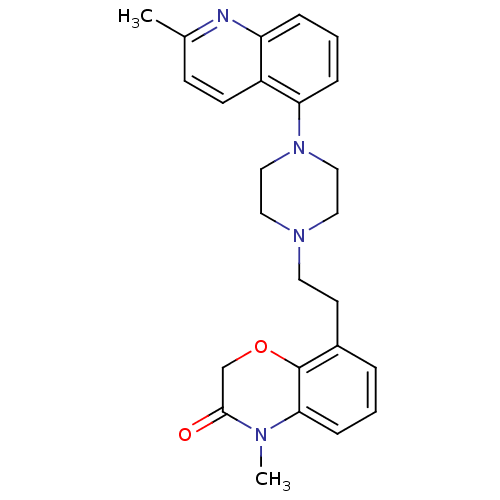
(CHEMBL469345)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H28N4O2/c1-18-9-10-20-21(26-18)6-4-7-22(20)29-15-13-28(14-16-29)12-11-19-5-3-8-23-25(19)31-17-24(30)27(23)2/h3-10H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326143

(1-(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)e...)Show SMILES CC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H29N5O2/c1-19-9-10-22-23(30-19)6-4-7-24(22)32-15-13-31(14-16-32)12-11-21-5-3-8-25-28(21)35-17-26-27(20(2)34)29-18-33(25)26/h3-10,18H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326132

(CHEMBL1241912 | N-methyl-6-(2-(4-(2-methylquinolin...)Show SMILES CNC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H30N6O2/c1-19-9-10-21-22(31-19)6-4-7-23(21)33-15-13-32(14-16-33)12-11-20-5-3-8-24-27(20)36-17-25-26(28(35)29-2)30-18-34(24)25/h3-10,18H,11-17H2,1-2H3,(H,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326138

(CHEMBL1241640 | ethyl 6-(2-(4-(2-methylquinolin-5-...)Show SMILES CCOC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C29H31N5O3/c1-3-36-29(35)27-26-18-37-28-21(6-4-9-25(28)34(26)19-30-27)12-13-32-14-16-33(17-15-32)24-8-5-7-23-22(24)11-10-20(2)31-23/h4-11,19H,3,12-18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326141

(1-methyl-6-(2-(4-(2-methylquinolin-5-yl)piperazin-...)Show SMILES Cc1nnc2COc3c(CCN4CCN(CC4)c4cccc5nc(C)ccc45)cccc3-n12 Show InChI InChI=1S/C26H28N6O/c1-18-9-10-21-22(27-18)6-4-7-23(21)31-15-13-30(14-16-31)12-11-20-5-3-8-24-26(20)33-17-25-29-28-19(2)32(24)25/h3-10H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326131

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(N)=O)CC1 Show InChI InChI=1S/C27H28N6O2/c1-18-8-9-20-21(30-18)5-3-6-22(20)32-14-12-31(13-15-32)11-10-19-4-2-7-23-26(19)35-16-24-25(27(28)34)29-17-33(23)24/h2-9,17H,10-16H2,1H3,(H2,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326150

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCOCC2)CC1 Show InChI InChI=1S/C31H34N6O3/c1-22-8-9-24-25(33-22)5-3-6-26(24)35-14-12-34(13-15-35)11-10-23-4-2-7-27-30(23)40-20-28-29(32-21-37(27)28)31(38)36-16-18-39-19-17-36/h2-9,21H,10-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG by scintillation proximity assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326140

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2cncn-32)CC1 Show InChI InChI=1S/C26H27N5O/c1-19-8-9-22-23(28-19)5-3-6-24(22)30-14-12-29(13-15-30)11-10-20-4-2-7-25-26(20)32-17-21-16-27-18-31(21)25/h2-9,16,18H,10-15,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein/7-benzyloxyquinoline as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50326139

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2nnnn-32)CC1 Show InChI InChI=1S/C24H25N7O/c1-17-8-9-19-20(25-17)5-3-6-21(19)30-14-12-29(13-15-30)11-10-18-4-2-7-22-24(18)32-16-23-26-27-28-31(22)23/h2-9H,10-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326150

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCOCC2)CC1 Show InChI InChI=1S/C31H34N6O3/c1-22-8-9-24-25(33-22)5-3-6-26(24)35-14-12-34(13-15-35)11-10-23-4-2-7-27-30(23)40-20-28-29(32-21-37(27)28)31(38)36-16-18-39-19-17-36/h2-9,21H,10-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG tail current by patch clamp electrophysiology assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326132

(CHEMBL1241912 | N-methyl-6-(2-(4-(2-methylquinolin...)Show SMILES CNC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H30N6O2/c1-19-9-10-21-22(31-19)6-4-7-23(21)33-15-13-32(14-16-33)12-11-20-5-3-8-24-27(20)36-17-25-26(28(35)29-2)30-18-34(24)25/h3-10,18H,11-17H2,1-2H3,(H,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG tail current by patch clamp electrophysiology assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50326137

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C#N)CC1 Show InChI InChI=1S/C27H26N6O/c1-19-8-9-21-22(30-19)5-3-6-24(21)32-14-12-31(13-15-32)11-10-20-4-2-7-25-27(20)34-17-26-23(16-28)29-18-33(25)26/h2-9,18H,10-15,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50326134

(CHEMBL1242167 | N-cyclobutyl-6-(2-(4-(2-methylquin...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)NC2CCC2)CC1 Show InChI InChI=1S/C31H34N6O2/c1-21-11-12-24-25(33-21)8-4-9-26(24)36-17-15-35(16-18-36)14-13-22-5-2-10-27-30(22)39-19-28-29(32-20-37(27)28)31(38)34-23-6-3-7-23/h2,4-5,8-12,20,23H,3,6-7,13-19H2,1H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326132

(CHEMBL1241912 | N-methyl-6-(2-(4-(2-methylquinolin...)Show SMILES CNC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H30N6O2/c1-19-9-10-21-22(31-19)6-4-7-23(21)33-15-13-32(14-16-33)12-11-20-5-3-8-24-27(20)36-17-25-26(28(35)29-2)30-18-34(24)25/h3-10,18H,11-17H2,1-2H3,(H,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes measured after incubation |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50326136

(1-(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)e...)Show SMILES CO\N=C(/C)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C29H32N6O2/c1-20-10-11-23-24(31-20)7-5-8-25(23)34-16-14-33(15-17-34)13-12-22-6-4-9-26-29(22)37-18-27-28(21(2)32-36-3)30-19-35(26)27/h4-11,19H,12-18H2,1-3H3/b32-21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50326133

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2nccn-32)CC1 Show InChI InChI=1S/C26H27N5O/c1-19-8-9-21-22(28-19)5-3-6-23(21)30-16-14-29(15-17-30)12-10-20-4-2-7-24-26(20)32-18-25-27-11-13-31(24)25/h2-9,11,13H,10,12,14-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50326136

(1-(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)e...)Show SMILES CO\N=C(/C)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C29H32N6O2/c1-20-10-11-23-24(31-20)7-5-8-25(23)34-16-14-33(15-17-34)13-12-22-6-4-9-26-29(22)37-18-27-28(21(2)32-36-3)30-19-35(26)27/h4-11,19H,12-18H2,1-3H3/b32-21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50326135

(CHEMBL1242436 | azetidin-1-yl(6-(2-(4-(2-methylqui...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCC2)CC1 Show InChI InChI=1S/C30H32N6O2/c1-21-9-10-23-24(32-21)6-3-7-25(23)34-17-15-33(16-18-34)14-11-22-5-2-8-26-29(22)38-19-27-28(31-20-36(26)27)30(37)35-12-4-13-35/h2-3,5-10,20H,4,11-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326137

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C#N)CC1 Show InChI InChI=1S/C27H26N6O/c1-19-8-9-21-22(30-19)5-3-6-24(21)32-14-12-31(13-15-32)11-10-20-4-2-7-25-27(20)34-17-26-23(16-28)29-18-33(25)26/h2-9,18H,10-15,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein/7-benzyloxyquinoline as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50326133

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2nccn-32)CC1 Show InChI InChI=1S/C26H27N5O/c1-19-8-9-21-22(28-19)5-3-6-23(21)30-16-14-29(15-17-30)12-10-20-4-2-7-24-26(20)32-18-25-27-11-13-31(24)25/h2-9,11,13H,10,12,14-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50326138

(CHEMBL1241640 | ethyl 6-(2-(4-(2-methylquinolin-5-...)Show SMILES CCOC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C29H31N5O3/c1-3-36-29(35)27-26-18-37-28-21(6-4-9-25(28)34(26)19-30-27)12-13-32-14-16-33(17-15-32)24-8-5-7-23-22(24)11-10-20(2)31-23/h4-11,19H,3,12-18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50326143

(1-(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)e...)Show SMILES CC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H29N5O2/c1-19-9-10-22-23(30-19)6-4-7-24(22)32-15-13-31(14-16-32)12-11-21-5-3-8-25-28(21)35-17-26-27(20(2)34)29-18-33(25)26/h3-10,18H,11-17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYPD6 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326132

(CHEMBL1241912 | N-methyl-6-(2-(4-(2-methylquinolin...)Show SMILES CNC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H30N6O2/c1-19-9-10-21-22(31-19)6-4-7-23(21)33-15-13-32(14-16-33)12-11-20-5-3-8-24-27(20)36-17-25-26(28(35)29-2)30-18-34(24)25/h3-10,18H,11-17H2,1-2H3,(H,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes measured after incubation |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50326142

(3-methyl-6-(2-(4-(2-methylquinolin-5-yl)piperazin-...)Show SMILES Cc1nnn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C26H28N6O/c1-18-9-10-21-22(27-18)6-4-7-23(21)31-15-13-30(14-16-31)12-11-20-5-3-8-24-26(20)33-17-25-19(2)28-29-32(24)25/h3-10H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326142

(3-methyl-6-(2-(4-(2-methylquinolin-5-yl)piperazin-...)Show SMILES Cc1nnn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C26H28N6O/c1-18-9-10-21-22(27-18)6-4-7-23(21)31-15-13-30(14-16-31)12-11-20-5-3-8-24-26(20)33-17-25-19(2)28-29-32(24)25/h3-10H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein/7-benzyloxyquinoline as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50326143

(1-(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)e...)Show SMILES CC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H29N5O2/c1-19-9-10-22-23(30-19)6-4-7-24(22)32-15-13-31(14-16-32)12-11-21-5-3-8-25-28(21)35-17-26-27(20(2)34)29-18-33(25)26/h3-10,18H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50326142

(3-methyl-6-(2-(4-(2-methylquinolin-5-yl)piperazin-...)Show SMILES Cc1nnn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C26H28N6O/c1-18-9-10-21-22(27-18)6-4-7-23(21)31-15-13-30(14-16-31)12-11-20-5-3-8-24-26(20)33-17-25-19(2)28-29-32(24)25/h3-10H,11-17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYPD6 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50326142

(3-methyl-6-(2-(4-(2-methylquinolin-5-yl)piperazin-...)Show SMILES Cc1nnn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C26H28N6O/c1-18-9-10-21-22(27-18)6-4-7-23(21)31-15-13-30(14-16-31)12-11-20-5-3-8-24-26(20)33-17-25-19(2)28-29-32(24)25/h3-10H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50326143

(1-(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)e...)Show SMILES CC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H29N5O2/c1-19-9-10-22-23(30-19)6-4-7-24(22)32-15-13-31(14-16-32)12-11-21-5-3-8-25-28(21)35-17-26-27(20(2)34)29-18-33(25)26/h3-10,18H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326142

(3-methyl-6-(2-(4-(2-methylquinolin-5-yl)piperazin-...)Show SMILES Cc1nnn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C26H28N6O/c1-18-9-10-21-22(27-18)6-4-7-23(21)31-15-13-30(14-16-31)12-11-20-5-3-8-24-26(20)33-17-25-19(2)28-29-32(24)25/h3-10H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50326143

(1-(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)e...)Show SMILES CC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H29N5O2/c1-19-9-10-22-23(30-19)6-4-7-24(22)32-15-13-31(14-16-32)12-11-21-5-3-8-25-28(21)35-17-26-27(20(2)34)29-18-33(25)26/h3-10,18H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326143

(1-(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)e...)Show SMILES CC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H29N5O2/c1-19-9-10-22-23(30-19)6-4-7-24(22)32-15-13-31(14-16-32)12-11-21-5-3-8-25-28(21)35-17-26-27(20(2)34)29-18-33(25)26/h3-10,18H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326143

(1-(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)e...)Show SMILES CC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H29N5O2/c1-19-9-10-22-23(30-19)6-4-7-24(22)32-15-13-31(14-16-32)12-11-21-5-3-8-25-28(21)35-17-26-27(20(2)34)29-18-33(25)26/h3-10,18H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein/7-benzyloxyquinoline as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50326142

(3-methyl-6-(2-(4-(2-methylquinolin-5-yl)piperazin-...)Show SMILES Cc1nnn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C26H28N6O/c1-18-9-10-21-22(27-18)6-4-7-23(21)31-15-13-30(14-16-31)12-11-20-5-3-8-24-26(20)33-17-25-19(2)28-29-32(24)25/h3-10H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326132

(CHEMBL1241912 | N-methyl-6-(2-(4-(2-methylquinolin...)Show SMILES CNC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H30N6O2/c1-19-9-10-21-22(31-19)6-4-7-23(21)33-15-13-32(14-16-33)12-11-20-5-3-8-24-27(20)36-17-25-26(28(35)29-2)30-18-34(24)25/h3-10,18H,11-17H2,1-2H3,(H,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes measured after incubation |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50326148

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCCC2)CC1 Show InChI InChI=1S/C31H34N6O2/c1-22-10-11-24-25(33-22)7-5-8-26(24)35-18-16-34(17-19-35)15-12-23-6-4-9-27-30(23)39-20-28-29(32-21-37(27)28)31(38)36-13-2-3-14-36/h4-11,21H,2-3,12-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326148

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCCC2)CC1 Show InChI InChI=1S/C31H34N6O2/c1-22-10-11-24-25(33-22)7-5-8-26(24)35-18-16-34(17-19-35)15-12-23-6-4-9-27-30(23)39-20-28-29(32-21-37(27)28)31(38)36-13-2-3-14-36/h4-11,21H,2-3,12-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50326148

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCCC2)CC1 Show InChI InChI=1S/C31H34N6O2/c1-22-10-11-24-25(33-22)7-5-8-26(24)35-18-16-34(17-19-35)15-12-23-6-4-9-27-30(23)39-20-28-29(32-21-37(27)28)31(38)36-13-2-3-14-36/h4-11,21H,2-3,12-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50326148

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCCC2)CC1 Show InChI InChI=1S/C31H34N6O2/c1-22-10-11-24-25(33-22)7-5-8-26(24)35-18-16-34(17-19-35)15-12-23-6-4-9-27-30(23)39-20-28-29(32-21-37(27)28)31(38)36-13-2-3-14-36/h4-11,21H,2-3,12-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50326148

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCCC2)CC1 Show InChI InChI=1S/C31H34N6O2/c1-22-10-11-24-25(33-22)7-5-8-26(24)35-18-16-34(17-19-35)15-12-23-6-4-9-27-30(23)39-20-28-29(32-21-37(27)28)31(38)36-13-2-3-14-36/h4-11,21H,2-3,12-20H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYPD6 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326148

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCCC2)CC1 Show InChI InChI=1S/C31H34N6O2/c1-22-10-11-24-25(33-22)7-5-8-26(24)35-18-16-34(17-19-35)15-12-23-6-4-9-27-30(23)39-20-28-29(32-21-37(27)28)31(38)36-13-2-3-14-36/h4-11,21H,2-3,12-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein/7-benzyloxyquinoline as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50326146

(CHEMBL1242081 | N-cyclopropyl-6-(2-(4-(2-methylqui...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)NC2CC2)CC1 Show InChI InChI=1S/C30H32N6O2/c1-20-8-11-23-24(32-20)5-3-6-25(23)35-16-14-34(15-17-35)13-12-21-4-2-7-26-29(21)38-18-27-28(31-19-36(26)27)30(37)33-22-9-10-22/h2-8,11,19,22H,9-10,12-18H2,1H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50326146

(CHEMBL1242081 | N-cyclopropyl-6-(2-(4-(2-methylqui...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)NC2CC2)CC1 Show InChI InChI=1S/C30H32N6O2/c1-20-8-11-23-24(32-20)5-3-6-25(23)35-16-14-34(15-17-35)13-12-21-4-2-7-26-29(21)38-18-27-28(31-19-36(26)27)30(37)33-22-9-10-22/h2-8,11,19,22H,9-10,12-18H2,1H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326146

(CHEMBL1242081 | N-cyclopropyl-6-(2-(4-(2-methylqui...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)NC2CC2)CC1 Show InChI InChI=1S/C30H32N6O2/c1-20-8-11-23-24(32-20)5-3-6-25(23)35-16-14-34(15-17-35)13-12-21-4-2-7-26-29(21)38-18-27-28(31-19-36(26)27)30(37)33-22-9-10-22/h2-8,11,19,22H,9-10,12-18H2,1H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein/7-benzyloxyquinoline as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50326146

(CHEMBL1242081 | N-cyclopropyl-6-(2-(4-(2-methylqui...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)NC2CC2)CC1 Show InChI InChI=1S/C30H32N6O2/c1-20-8-11-23-24(32-20)5-3-6-25(23)35-16-14-34(15-17-35)13-12-21-4-2-7-26-29(21)38-18-27-28(31-19-36(26)27)30(37)33-22-9-10-22/h2-8,11,19,22H,9-10,12-18H2,1H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326146

(CHEMBL1242081 | N-cyclopropyl-6-(2-(4-(2-methylqui...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)NC2CC2)CC1 Show InChI InChI=1S/C30H32N6O2/c1-20-8-11-23-24(32-20)5-3-6-25(23)35-16-14-34(15-17-35)13-12-21-4-2-7-26-29(21)38-18-27-28(31-19-36(26)27)30(37)33-22-9-10-22/h2-8,11,19,22H,9-10,12-18H2,1H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50326146

(CHEMBL1242081 | N-cyclopropyl-6-(2-(4-(2-methylqui...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)NC2CC2)CC1 Show InChI InChI=1S/C30H32N6O2/c1-20-8-11-23-24(32-20)5-3-6-25(23)35-16-14-34(15-17-35)13-12-21-4-2-7-26-29(21)38-18-27-28(31-19-36(26)27)30(37)33-22-9-10-22/h2-8,11,19,22H,9-10,12-18H2,1H3,(H,33,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYPD6 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326132

(CHEMBL1241912 | N-methyl-6-(2-(4-(2-methylquinolin...)Show SMILES CNC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H30N6O2/c1-19-9-10-21-22(31-19)6-4-7-23(21)33-15-13-32(14-16-33)12-11-20-5-3-8-24-27(20)36-17-25-26(28(35)29-2)30-18-34(24)25/h3-10,18H,11-17H2,1-2H3,(H,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes measured immediately |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50326145

(CHEMBL1241998 | N-isopropyl-6-(2-(4-(2-methylquino...)Show SMILES CC(C)NC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C30H34N6O2/c1-20(2)32-30(37)28-27-18-38-29-22(6-4-9-26(29)36(27)19-31-28)12-13-34-14-16-35(17-15-34)25-8-5-7-24-23(25)11-10-21(3)33-24/h4-11,19-20H,12-18H2,1-3H3,(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326145

(CHEMBL1241998 | N-isopropyl-6-(2-(4-(2-methylquino...)Show SMILES CC(C)NC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C30H34N6O2/c1-20(2)32-30(37)28-27-18-38-29-22(6-4-9-26(29)36(27)19-31-28)12-13-34-14-16-35(17-15-34)25-8-5-7-24-23(25)11-10-21(3)33-24/h4-11,19-20H,12-18H2,1-3H3,(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein/7-benzyloxyquinoline as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50326145

(CHEMBL1241998 | N-isopropyl-6-(2-(4-(2-methylquino...)Show SMILES CC(C)NC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C30H34N6O2/c1-20(2)32-30(37)28-27-18-38-29-22(6-4-9-26(29)36(27)19-31-28)12-13-34-14-16-35(17-15-34)25-8-5-7-24-23(25)11-10-21(3)33-24/h4-11,19-20H,12-18H2,1-3H3,(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50326145

(CHEMBL1241998 | N-isopropyl-6-(2-(4-(2-methylquino...)Show SMILES CC(C)NC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C30H34N6O2/c1-20(2)32-30(37)28-27-18-38-29-22(6-4-9-26(29)36(27)19-31-28)12-13-34-14-16-35(17-15-34)25-8-5-7-24-23(25)11-10-21(3)33-24/h4-11,19-20H,12-18H2,1-3H3,(H,32,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYPD6 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50326145

(CHEMBL1241998 | N-isopropyl-6-(2-(4-(2-methylquino...)Show SMILES CC(C)NC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C30H34N6O2/c1-20(2)32-30(37)28-27-18-38-29-22(6-4-9-26(29)36(27)19-31-28)12-13-34-14-16-35(17-15-34)25-8-5-7-24-23(25)11-10-21(3)33-24/h4-11,19-20H,12-18H2,1-3H3,(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326145

(CHEMBL1241998 | N-isopropyl-6-(2-(4-(2-methylquino...)Show SMILES CC(C)NC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C30H34N6O2/c1-20(2)32-30(37)28-27-18-38-29-22(6-4-9-26(29)36(27)19-31-28)12-13-34-14-16-35(17-15-34)25-8-5-7-24-23(25)11-10-21(3)33-24/h4-11,19-20H,12-18H2,1-3H3,(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326131

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(N)=O)CC1 Show InChI InChI=1S/C27H28N6O2/c1-18-8-9-20-21(30-18)5-3-6-22(20)32-14-12-31(13-15-32)11-10-19-4-2-7-23-26(19)35-16-24-25(27(28)34)29-17-33(23)24/h2-9,17H,10-16H2,1H3,(H2,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326132

(CHEMBL1241912 | N-methyl-6-(2-(4-(2-methylquinolin...)Show SMILES CNC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H30N6O2/c1-19-9-10-21-22(31-19)6-4-7-23(21)33-15-13-32(14-16-33)12-11-20-5-3-8-24-27(20)36-17-25-26(28(35)29-2)30-18-34(24)25/h3-10,18H,11-17H2,1-2H3,(H,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50326131

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(N)=O)CC1 Show InChI InChI=1S/C27H28N6O2/c1-18-8-9-20-21(30-18)5-3-6-22(20)32-14-12-31(13-15-32)11-10-19-4-2-7-23-26(19)35-16-24-25(27(28)34)29-17-33(23)24/h2-9,17H,10-16H2,1H3,(H2,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50326132

(CHEMBL1241912 | N-methyl-6-(2-(4-(2-methylquinolin...)Show SMILES CNC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H30N6O2/c1-19-9-10-21-22(31-19)6-4-7-23(21)33-15-13-32(14-16-33)12-11-20-5-3-8-24-27(20)36-17-25-26(28(35)29-2)30-18-34(24)25/h3-10,18H,11-17H2,1-2H3,(H,29,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYPD6 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326131

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(N)=O)CC1 Show InChI InChI=1S/C27H28N6O2/c1-18-8-9-20-21(30-18)5-3-6-22(20)32-14-12-31(13-15-32)11-10-19-4-2-7-23-26(19)35-16-24-25(27(28)34)29-17-33(23)24/h2-9,17H,10-16H2,1H3,(H2,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein/7-benzyloxyquinoline as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50326132

(CHEMBL1241912 | N-methyl-6-(2-(4-(2-methylquinolin...)Show SMILES CNC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H30N6O2/c1-19-9-10-21-22(31-19)6-4-7-23(21)33-15-13-32(14-16-33)12-11-20-5-3-8-24-27(20)36-17-25-26(28(35)29-2)30-18-34(24)25/h3-10,18H,11-17H2,1-2H3,(H,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50326131

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(N)=O)CC1 Show InChI InChI=1S/C27H28N6O2/c1-18-8-9-20-21(30-18)5-3-6-22(20)32-14-12-31(13-15-32)11-10-19-4-2-7-23-26(19)35-16-24-25(27(28)34)29-17-33(23)24/h2-9,17H,10-16H2,1H3,(H2,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50326131

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(N)=O)CC1 Show InChI InChI=1S/C27H28N6O2/c1-18-8-9-20-21(30-18)5-3-6-22(20)32-14-12-31(13-15-32)11-10-19-4-2-7-23-26(19)35-16-24-25(27(28)34)29-17-33(23)24/h2-9,17H,10-16H2,1H3,(H2,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326132

(CHEMBL1241912 | N-methyl-6-(2-(4-(2-methylquinolin...)Show SMILES CNC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H30N6O2/c1-19-9-10-21-22(31-19)6-4-7-23(21)33-15-13-32(14-16-33)12-11-20-5-3-8-24-27(20)36-17-25-26(28(35)29-2)30-18-34(24)25/h3-10,18H,11-17H2,1-2H3,(H,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein/7-benzyloxyquinoline as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50326132

(CHEMBL1241912 | N-methyl-6-(2-(4-(2-methylquinolin...)Show SMILES CNC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H30N6O2/c1-19-9-10-21-22(31-19)6-4-7-23(21)33-15-13-32(14-16-33)12-11-20-5-3-8-24-27(20)36-17-25-26(28(35)29-2)30-18-34(24)25/h3-10,18H,11-17H2,1-2H3,(H,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50326131

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(N)=O)CC1 Show InChI InChI=1S/C27H28N6O2/c1-18-8-9-20-21(30-18)5-3-6-22(20)32-14-12-31(13-15-32)11-10-19-4-2-7-23-26(19)35-16-24-25(27(28)34)29-17-33(23)24/h2-9,17H,10-16H2,1H3,(H2,28,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYPD6 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50326132

(CHEMBL1241912 | N-methyl-6-(2-(4-(2-methylquinolin...)Show SMILES CNC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H30N6O2/c1-19-9-10-21-22(31-19)6-4-7-23(21)33-15-13-32(14-16-33)12-11-20-5-3-8-24-27(20)36-17-25-26(28(35)29-2)30-18-34(24)25/h3-10,18H,11-17H2,1-2H3,(H,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50326149

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCCCC2)CC1 Show InChI InChI=1S/C32H36N6O2/c1-23-11-12-25-26(34-23)8-6-9-27(25)36-19-17-35(18-20-36)16-13-24-7-5-10-28-31(24)40-21-29-30(33-22-38(28)29)32(39)37-14-3-2-4-15-37/h5-12,22H,2-4,13-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50326149

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCCCC2)CC1 Show InChI InChI=1S/C32H36N6O2/c1-23-11-12-25-26(34-23)8-6-9-27(25)36-19-17-35(18-20-36)16-13-24-7-5-10-28-31(24)40-21-29-30(33-22-38(28)29)32(39)37-14-3-2-4-15-37/h5-12,22H,2-4,13-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50326141

(1-methyl-6-(2-(4-(2-methylquinolin-5-yl)piperazin-...)Show SMILES Cc1nnc2COc3c(CCN4CCN(CC4)c4cccc5nc(C)ccc45)cccc3-n12 Show InChI InChI=1S/C26H28N6O/c1-18-9-10-21-22(27-18)6-4-7-23(21)31-15-13-30(14-16-31)12-11-20-5-3-8-24-26(20)33-17-25-29-28-19(2)32(24)25/h3-10H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50326141

(1-methyl-6-(2-(4-(2-methylquinolin-5-yl)piperazin-...)Show SMILES Cc1nnc2COc3c(CCN4CCN(CC4)c4cccc5nc(C)ccc45)cccc3-n12 Show InChI InChI=1S/C26H28N6O/c1-18-9-10-21-22(27-18)6-4-7-23(21)31-15-13-30(14-16-31)12-11-20-5-3-8-24-26(20)33-17-25-29-28-19(2)32(24)25/h3-10H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50326144

(CHEMBL1241824 | N,N-dimethyl-6-(2-(4-(2-methylquin...)Show SMILES CN(C)C(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C29H32N6O2/c1-20-10-11-22-23(31-20)7-5-8-24(22)34-16-14-33(15-17-34)13-12-21-6-4-9-25-28(21)37-18-26-27(29(36)32(2)3)30-19-35(25)26/h4-11,19H,12-18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326144

(CHEMBL1241824 | N,N-dimethyl-6-(2-(4-(2-methylquin...)Show SMILES CN(C)C(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C29H32N6O2/c1-20-10-11-22-23(31-20)7-5-8-24(22)34-16-14-33(15-17-34)13-12-21-6-4-9-25-28(21)37-18-26-27(29(36)32(2)3)30-19-35(25)26/h4-11,19H,12-18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50326144

(CHEMBL1241824 | N,N-dimethyl-6-(2-(4-(2-methylquin...)Show SMILES CN(C)C(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C29H32N6O2/c1-20-10-11-22-23(31-20)7-5-8-24(22)34-16-14-33(15-17-34)13-12-21-6-4-9-25-28(21)37-18-26-27(29(36)32(2)3)30-19-35(25)26/h4-11,19H,12-18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50326147

(CHEMBL1242345 | N-(cyclopropylmethyl)-6-(2-(4-(2-m...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)NCC2CC2)CC1 Show InChI InChI=1S/C31H34N6O2/c1-21-8-11-24-25(34-21)5-3-6-26(24)36-16-14-35(15-17-36)13-12-23-4-2-7-27-30(23)39-19-28-29(33-20-37(27)28)31(38)32-18-22-9-10-22/h2-8,11,20,22H,9-10,12-19H2,1H3,(H,32,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326147

(CHEMBL1242345 | N-(cyclopropylmethyl)-6-(2-(4-(2-m...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)NCC2CC2)CC1 Show InChI InChI=1S/C31H34N6O2/c1-21-8-11-24-25(34-21)5-3-6-26(24)36-16-14-35(15-17-36)13-12-23-4-2-7-27-30(23)39-19-28-29(33-20-37(27)28)31(38)32-18-22-9-10-22/h2-8,11,20,22H,9-10,12-19H2,1H3,(H,32,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326149

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCCCC2)CC1 Show InChI InChI=1S/C32H36N6O2/c1-23-11-12-25-26(34-23)8-6-9-27(25)36-19-17-35(18-20-36)16-13-24-7-5-10-28-31(24)40-21-29-30(33-22-38(28)29)32(39)37-14-3-2-4-15-37/h5-12,22H,2-4,13-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326150

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCOCC2)CC1 Show InChI InChI=1S/C31H34N6O3/c1-22-8-9-24-25(33-22)5-3-6-26(24)35-14-12-34(13-15-35)11-10-23-4-2-7-27-30(23)40-20-28-29(32-21-37(27)28)31(38)36-16-18-39-19-17-36/h2-9,21H,10-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50326144

(CHEMBL1241824 | N,N-dimethyl-6-(2-(4-(2-methylquin...)Show SMILES CN(C)C(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C29H32N6O2/c1-20-10-11-22-23(31-20)7-5-8-24(22)34-16-14-33(15-17-34)13-12-21-6-4-9-25-28(21)37-18-26-27(29(36)32(2)3)30-19-35(25)26/h4-11,19H,12-18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50326147

(CHEMBL1242345 | N-(cyclopropylmethyl)-6-(2-(4-(2-m...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)NCC2CC2)CC1 Show InChI InChI=1S/C31H34N6O2/c1-21-8-11-24-25(34-21)5-3-6-26(24)36-16-14-35(15-17-36)13-12-23-4-2-7-27-30(23)39-19-28-29(33-20-37(27)28)31(38)32-18-22-9-10-22/h2-8,11,20,22H,9-10,12-19H2,1H3,(H,32,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYPD6 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50326149

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCCCC2)CC1 Show InChI InChI=1S/C32H36N6O2/c1-23-11-12-25-26(34-23)8-6-9-27(25)36-19-17-35(18-20-36)16-13-24-7-5-10-28-31(24)40-21-29-30(33-22-38(28)29)32(39)37-14-3-2-4-15-37/h5-12,22H,2-4,13-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50326149

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCCCC2)CC1 Show InChI InChI=1S/C32H36N6O2/c1-23-11-12-25-26(34-23)8-6-9-27(25)36-19-17-35(18-20-36)16-13-24-7-5-10-28-31(24)40-21-29-30(33-22-38(28)29)32(39)37-14-3-2-4-15-37/h5-12,22H,2-4,13-21H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYPD6 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326149

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCCCC2)CC1 Show InChI InChI=1S/C32H36N6O2/c1-23-11-12-25-26(34-23)8-6-9-27(25)36-19-17-35(18-20-36)16-13-24-7-5-10-28-31(24)40-21-29-30(33-22-38(28)29)32(39)37-14-3-2-4-15-37/h5-12,22H,2-4,13-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein/7-benzyloxyquinoline as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50326150

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCOCC2)CC1 Show InChI InChI=1S/C31H34N6O3/c1-22-8-9-24-25(33-22)5-3-6-26(24)35-14-12-34(13-15-35)11-10-23-4-2-7-27-30(23)40-20-28-29(32-21-37(27)28)31(38)36-16-18-39-19-17-36/h2-9,21H,10-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50326131

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(N)=O)CC1 Show InChI InChI=1S/C27H28N6O2/c1-18-8-9-20-21(30-18)5-3-6-22(20)32-14-12-31(13-15-32)11-10-19-4-2-7-23-26(19)35-16-24-25(27(28)34)29-17-33(23)24/h2-9,17H,10-16H2,1H3,(H2,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG tail current by patch clamp electrophysiology assay |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326141

(1-methyl-6-(2-(4-(2-methylquinolin-5-yl)piperazin-...)Show SMILES Cc1nnc2COc3c(CCN4CCN(CC4)c4cccc5nc(C)ccc45)cccc3-n12 Show InChI InChI=1S/C26H28N6O/c1-18-9-10-21-22(27-18)6-4-7-23(21)31-15-13-30(14-16-31)12-11-20-5-3-8-24-26(20)33-17-25-29-28-19(2)32(24)25/h3-10H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50326141

(1-methyl-6-(2-(4-(2-methylquinolin-5-yl)piperazin-...)Show SMILES Cc1nnc2COc3c(CCN4CCN(CC4)c4cccc5nc(C)ccc45)cccc3-n12 Show InChI InChI=1S/C26H28N6O/c1-18-9-10-21-22(27-18)6-4-7-23(21)31-15-13-30(14-16-31)12-11-20-5-3-8-24-26(20)33-17-25-29-28-19(2)32(24)25/h3-10H,11-17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYPD6 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50326150

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCOCC2)CC1 Show InChI InChI=1S/C31H34N6O3/c1-22-8-9-24-25(33-22)5-3-6-26(24)35-14-12-34(13-15-35)11-10-23-4-2-7-27-30(23)40-20-28-29(32-21-37(27)28)31(38)36-16-18-39-19-17-36/h2-9,21H,10-20H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYPD6 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50326150

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCOCC2)CC1 Show InChI InChI=1S/C31H34N6O3/c1-22-8-9-24-25(33-22)5-3-6-26(24)35-14-12-34(13-15-35)11-10-23-4-2-7-27-30(23)40-20-28-29(32-21-37(27)28)31(38)36-16-18-39-19-17-36/h2-9,21H,10-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50326144

(CHEMBL1241824 | N,N-dimethyl-6-(2-(4-(2-methylquin...)Show SMILES CN(C)C(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C29H32N6O2/c1-20-10-11-22-23(31-20)7-5-8-24(22)34-16-14-33(15-17-34)13-12-21-6-4-9-25-28(21)37-18-26-27(29(36)32(2)3)30-19-35(25)26/h4-11,19H,12-18H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYPD6 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326144

(CHEMBL1241824 | N,N-dimethyl-6-(2-(4-(2-methylquin...)Show SMILES CN(C)C(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C29H32N6O2/c1-20-10-11-22-23(31-20)7-5-8-24(22)34-16-14-33(15-17-34)13-12-21-6-4-9-25-28(21)37-18-26-27(29(36)32(2)3)30-19-35(25)26/h4-11,19H,12-18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein/7-benzyloxyquinoline as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50326150

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCOCC2)CC1 Show InChI InChI=1S/C31H34N6O3/c1-22-8-9-24-25(33-22)5-3-6-26(24)35-14-12-34(13-15-35)11-10-23-4-2-7-27-30(23)40-20-28-29(32-21-37(27)28)31(38)36-16-18-39-19-17-36/h2-9,21H,10-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326150

((6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)eth...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)N2CCOCC2)CC1 Show InChI InChI=1S/C31H34N6O3/c1-22-8-9-24-25(33-22)5-3-6-26(24)35-14-12-34(13-15-35)11-10-23-4-2-7-27-30(23)40-20-28-29(32-21-37(27)28)31(38)36-16-18-39-19-17-36/h2-9,21H,10-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein/7-benzyloxyquinoline as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50326141

(1-methyl-6-(2-(4-(2-methylquinolin-5-yl)piperazin-...)Show SMILES Cc1nnc2COc3c(CCN4CCN(CC4)c4cccc5nc(C)ccc45)cccc3-n12 Show InChI InChI=1S/C26H28N6O/c1-18-9-10-21-22(27-18)6-4-7-23(21)31-15-13-30(14-16-31)12-11-20-5-3-8-24-26(20)33-17-25-29-28-19(2)32(24)25/h3-10H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326141

(1-methyl-6-(2-(4-(2-methylquinolin-5-yl)piperazin-...)Show SMILES Cc1nnc2COc3c(CCN4CCN(CC4)c4cccc5nc(C)ccc45)cccc3-n12 Show InChI InChI=1S/C26H28N6O/c1-18-9-10-21-22(27-18)6-4-7-23(21)31-15-13-30(14-16-31)12-11-20-5-3-8-24-26(20)33-17-25-29-28-19(2)32(24)25/h3-10H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein/7-benzyloxyquinoline as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50326147

(CHEMBL1242345 | N-(cyclopropylmethyl)-6-(2-(4-(2-m...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)NCC2CC2)CC1 Show InChI InChI=1S/C31H34N6O2/c1-21-8-11-24-25(34-21)5-3-6-26(24)36-16-14-35(15-17-36)13-12-23-4-2-7-27-30(23)39-19-28-29(33-20-37(27)28)31(38)32-18-22-9-10-22/h2-8,11,20,22H,9-10,12-19H2,1H3,(H,32,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50326147

(CHEMBL1242345 | N-(cyclopropylmethyl)-6-(2-(4-(2-m...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)NCC2CC2)CC1 Show InChI InChI=1S/C31H34N6O2/c1-21-8-11-24-25(34-21)5-3-6-26(24)36-16-14-35(15-17-36)13-12-23-4-2-7-27-30(23)39-19-28-29(33-20-37(27)28)31(38)32-18-22-9-10-22/h2-8,11,20,22H,9-10,12-19H2,1H3,(H,32,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 preincubated for 10 mins |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326147

(CHEMBL1242345 | N-(cyclopropylmethyl)-6-(2-(4-(2-m...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(=O)NCC2CC2)CC1 Show InChI InChI=1S/C31H34N6O2/c1-21-8-11-24-25(34-21)5-3-6-26(24)36-16-14-35(15-17-36)13-12-23-4-2-7-27-30(23)39-19-28-29(33-20-37(27)28)31(38)32-18-22-9-10-22/h2-8,11,20,22H,9-10,12-19H2,1H3,(H,32,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using diethoxyflourescein/7-benzyloxyquinoline as substrate |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326132

(CHEMBL1241912 | N-methyl-6-(2-(4-(2-methylquinolin...)Show SMILES CNC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H30N6O2/c1-19-9-10-21-22(31-19)6-4-7-23(21)33-15-13-32(14-16-33)12-11-20-5-3-8-24-27(20)36-17-25-26(28(35)29-2)30-18-34(24)25/h3-10,18H,11-17H2,1-2H3,(H,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes measured immediately |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326132

(CHEMBL1241912 | N-methyl-6-(2-(4-(2-methylquinolin...)Show SMILES CNC(=O)c1ncn-2c1COc1c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc-21 Show InChI InChI=1S/C28H30N6O2/c1-19-9-10-21-22(31-19)6-4-7-23(21)33-15-13-32(14-16-33)12-11-20-5-3-8-24-27(20)36-17-25-26(28(35)29-2)30-18-34(24)25/h3-10,18H,11-17H2,1-2H3,(H,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes measured immediately |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326131

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(N)=O)CC1 Show InChI InChI=1S/C27H28N6O2/c1-18-8-9-20-21(30-18)5-3-6-22(20)32-14-12-31(13-15-32)11-10-19-4-2-7-23-26(19)35-16-24-25(27(28)34)29-17-33(23)24/h2-9,17H,10-16H2,1H3,(H2,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes measured after incubation |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326131

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(N)=O)CC1 Show InChI InChI=1S/C27H28N6O2/c1-18-8-9-20-21(30-18)5-3-6-22(20)32-14-12-31(13-15-32)11-10-19-4-2-7-23-26(19)35-16-24-25(27(28)34)29-17-33(23)24/h2-9,17H,10-16H2,1H3,(H2,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes measured after incubation |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326131

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(N)=O)CC1 Show InChI InChI=1S/C27H28N6O2/c1-18-8-9-20-21(30-18)5-3-6-22(20)32-14-12-31(13-15-32)11-10-19-4-2-7-23-26(19)35-16-24-25(27(28)34)29-17-33(23)24/h2-9,17H,10-16H2,1H3,(H2,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes measured immediately |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50326131

(6-(2-(4-(2-methylquinolin-5-yl)piperazin-1-yl)ethy...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc-3c2OCc2c(ncn-32)C(N)=O)CC1 Show InChI InChI=1S/C27H28N6O2/c1-18-8-9-20-21(30-18)5-3-6-22(20)32-14-12-31(13-15-32)11-10-19-4-2-7-23-26(19)35-16-24-25(27(28)34)29-17-33(23)24/h2-9,17H,10-16H2,1H3,(H2,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes measured immediately |
J Med Chem 53: 5827-43 (2010)
Article DOI: 10.1021/jm100482n
BindingDB Entry DOI: 10.7270/Q2D50N6M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data