 Found 54 hits of Enzyme Inhibition Constant Data
Found 54 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50335818
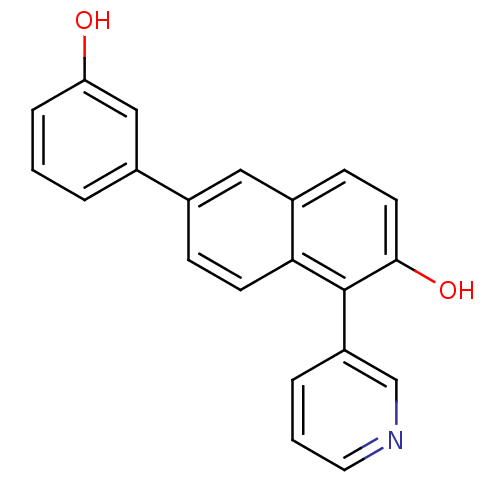
(6-(3-Hydroxyphenyl)-1-(pyridin-3-yl)-2-naphthol | ...)Show InChI InChI=1S/C21H15NO2/c23-18-5-1-3-14(12-18)15-6-8-19-16(11-15)7-9-20(24)21(19)17-4-2-10-22-13-17/h1-13,23-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50335815
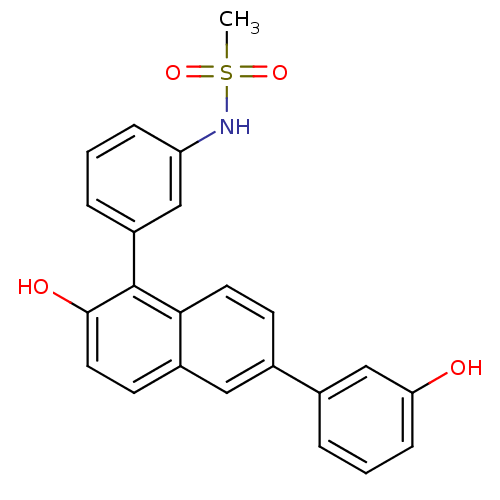
(CHEMBL1650658 | N-{3-[2-Hydroxy-6-(3-hydroxyphenyl...)Show SMILES CS(=O)(=O)Nc1cccc(c1)-c1c(O)ccc2cc(ccc12)-c1cccc(O)c1 Show InChI InChI=1S/C23H19NO4S/c1-29(27,28)24-19-6-2-5-18(13-19)23-21-10-8-16(12-17(21)9-11-22(23)26)15-4-3-7-20(25)14-15/h2-14,24-26H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E1 from human placental 17beta-HSD1 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50261942

(6-(3-Hydroxyphenyl)-1-phenyl-2-naphthol | CHEMBL46...)Show InChI InChI=1S/C22H16O2/c23-19-8-4-7-16(14-19)17-9-11-20-18(13-17)10-12-21(24)22(20)15-5-2-1-3-6-15/h1-14,23-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E1 from human placental 17beta-HSD1 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50335818

(6-(3-Hydroxyphenyl)-1-(pyridin-3-yl)-2-naphthol | ...)Show InChI InChI=1S/C21H15NO2/c23-18-5-1-3-14(12-18)15-6-8-19-16(11-15)7-9-20(24)21(19)17-4-2-10-22-13-17/h1-13,23-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E1 from human placental 17beta-HSD1 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50335817
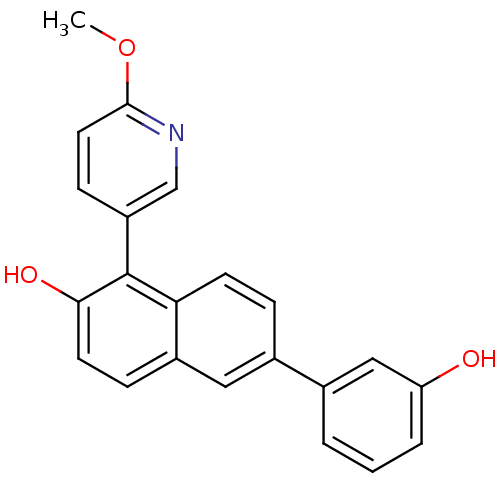
(6-(3-hydroxyphenyl)-1-(6-methoxypyridin-3-yl)napht...)Show SMILES COc1ccc(cn1)-c1c(O)ccc2cc(ccc12)-c1cccc(O)c1 Show InChI InChI=1S/C22H17NO3/c1-26-21-10-7-17(13-23-21)22-19-8-5-15(11-16(19)6-9-20(22)25)14-3-2-4-18(24)12-14/h2-13,24-25H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E1 from human placental 17beta-HSD1 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50335816

(1,6-Bis(3-hydroxyphenyl)-2-naphthol | CHEMBL165065...)Show SMILES Oc1cccc(c1)-c1ccc2c(c(O)ccc2c1)-c1cccc(O)c1 Show InChI InChI=1S/C22H16O3/c23-18-5-1-3-14(12-18)15-7-9-20-16(11-15)8-10-21(25)22(20)17-4-2-6-19(24)13-17/h1-13,23-25H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E1 from human placental 17beta-HSD1 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50335816

(1,6-Bis(3-hydroxyphenyl)-2-naphthol | CHEMBL165065...)Show SMILES Oc1cccc(c1)-c1ccc2c(c(O)ccc2c1)-c1cccc(O)c1 Show InChI InChI=1S/C22H16O3/c23-18-5-1-3-14(12-18)15-7-9-20-16(11-15)8-10-21(25)22(20)17-4-2-6-19(24)13-17/h1-13,23-25H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50335819

(1-(3-Aminophenyl)-6-(3-hydroxyphenyl)-2-naphthol |...)Show SMILES Nc1cccc(c1)-c1c(O)ccc2cc(ccc12)-c1cccc(O)c1 Show InChI InChI=1S/C22H17NO2/c23-18-5-1-4-17(12-18)22-20-9-7-15(11-16(20)8-10-21(22)25)14-3-2-6-19(24)13-14/h1-13,24-25H,23H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E1 from human placental 17beta-HSD1 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50335820
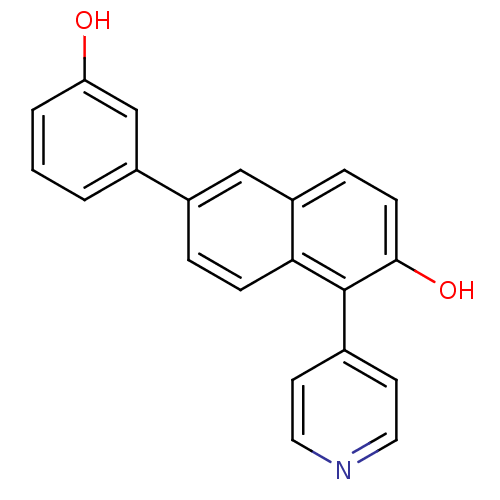
(6-(3-Hydroxyphenyl)-1-(pyridin-4-yl)-2-naphthol | ...)Show InChI InChI=1S/C21H15NO2/c23-18-3-1-2-15(13-18)16-4-6-19-17(12-16)5-7-20(24)21(19)14-8-10-22-11-9-14/h1-13,23-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E1 from human placental 17beta-HSD1 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50335821

(1-(3-Furyl)-6-(3-hydroxyphenyl)-2-naphthol | CHEMB...)Show InChI InChI=1S/C20H14O3/c21-17-3-1-2-13(11-17)14-4-6-18-15(10-14)5-7-19(22)20(18)16-8-9-23-12-16/h1-12,21-22H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E1 from human placental 17beta-HSD1 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50335815

(CHEMBL1650658 | N-{3-[2-Hydroxy-6-(3-hydroxyphenyl...)Show SMILES CS(=O)(=O)Nc1cccc(c1)-c1c(O)ccc2cc(ccc12)-c1cccc(O)c1 Show InChI InChI=1S/C23H19NO4S/c1-29(27,28)24-19-6-2-5-18(13-19)23-21-10-8-16(12-17(21)9-11-22(23)26)15-4-3-7-20(25)14-15/h2-14,24-26H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E1 from 17beta-HSD1 in human T47D cells after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50335816

(1,6-Bis(3-hydroxyphenyl)-2-naphthol | CHEMBL165065...)Show SMILES Oc1cccc(c1)-c1ccc2c(c(O)ccc2c1)-c1cccc(O)c1 Show InChI InChI=1S/C22H16O3/c23-18-5-1-3-14(12-18)15-7-9-20-16(11-15)8-10-21(25)22(20)17-4-2-6-19(24)13-17/h1-13,23-25H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E1 from 17beta-HSD1 in human T47D cells after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50168371
(6-(3-Hydroxy-phenyl)-naphthalen-2-ol | 6-(3-hydrox...)Show InChI InChI=1S/C16H12O2/c17-15-3-1-2-11(9-15)12-4-5-14-10-16(18)7-6-13(14)8-12/h1-10,17-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E1 from human placental 17beta-HSD1 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50335818

(6-(3-Hydroxyphenyl)-1-(pyridin-3-yl)-2-naphthol | ...)Show InChI InChI=1S/C21H15NO2/c23-18-5-1-3-14(12-18)15-6-8-19-16(11-15)7-9-20(24)21(19)17-4-2-10-22-13-17/h1-13,23-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50335818

(6-(3-Hydroxyphenyl)-1-(pyridin-3-yl)-2-naphthol | ...)Show InChI InChI=1S/C21H15NO2/c23-18-5-1-3-14(12-18)15-6-8-19-16(11-15)7-9-20(24)21(19)17-4-2-10-22-13-17/h1-13,23-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E1 from 17beta-HSD1 in human T47D cells after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50335818

(6-(3-Hydroxyphenyl)-1-(pyridin-3-yl)-2-naphthol | ...)Show InChI InChI=1S/C21H15NO2/c23-18-5-1-3-14(12-18)15-6-8-19-16(11-15)7-9-20(24)21(19)17-4-2-10-22-13-17/h1-13,23-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50168371

(6-(3-Hydroxy-phenyl)-naphthalen-2-ol | 6-(3-hydrox...)Show InChI InChI=1S/C16H12O2/c17-15-3-1-2-11(9-15)12-4-5-14-10-16(18)7-6-13(14)8-12/h1-10,17-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 229 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E1 from 17beta-HSD1 in human T47D cells after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50090677
(4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...)Show InChI InChI=1S/C15H14N4O2S/c16-12-6-8-14(9-7-12)22(20,21)18-15-10-11-17-19(15)13-4-2-1-3-5-13/h1-11,18H,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50335816

(1,6-Bis(3-hydroxyphenyl)-2-naphthol | CHEMBL165065...)Show SMILES Oc1cccc(c1)-c1ccc2c(c(O)ccc2c1)-c1cccc(O)c1 Show InChI InChI=1S/C22H16O3/c23-18-5-1-3-14(12-18)15-7-9-20-16(11-15)8-10-21(25)22(20)17-4-2-6-19(24)13-17/h1-13,23-25H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50261942

(6-(3-Hydroxyphenyl)-1-phenyl-2-naphthol | CHEMBL46...)Show InChI InChI=1S/C22H16O2/c23-19-8-4-7-16(14-19)17-9-11-20-18(13-17)10-12-21(24)22(20)15-5-2-1-3-6-15/h1-14,23-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 281 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E1 from 17beta-HSD1 in human T47D cells after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50335815

(CHEMBL1650658 | N-{3-[2-Hydroxy-6-(3-hydroxyphenyl...)Show SMILES CS(=O)(=O)Nc1cccc(c1)-c1c(O)ccc2cc(ccc12)-c1cccc(O)c1 Show InChI InChI=1S/C23H19NO4S/c1-29(27,28)24-19-6-2-5-18(13-19)23-21-10-8-16(12-17(21)9-11-22(23)26)15-4-3-7-20(25)14-15/h2-14,24-26H,1H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 403 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E2 from human placental 17beta-HSD2 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50335821

(1-(3-Furyl)-6-(3-hydroxyphenyl)-2-naphthol | CHEMB...)Show InChI InChI=1S/C20H14O3/c21-17-3-1-2-13(11-17)14-4-6-18-15(10-14)5-7-19(22)20(18)16-8-9-23-12-16/h1-12,21-22H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 527 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E2 from human placental 17beta-HSD2 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50335817

(6-(3-hydroxyphenyl)-1-(6-methoxypyridin-3-yl)napht...)Show SMILES COc1ccc(cn1)-c1c(O)ccc2cc(ccc12)-c1cccc(O)c1 Show InChI InChI=1S/C22H17NO3/c1-26-21-10-7-17(13-23-21)22-19-8-5-15(11-16(19)6-9-20(22)25)14-3-2-4-18(24)12-14/h2-13,24-25H,1H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E2 from human placental 17beta-HSD2 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50261942

(6-(3-Hydroxyphenyl)-1-phenyl-2-naphthol | CHEMBL46...)Show InChI InChI=1S/C22H16O2/c23-19-8-4-7-16(14-19)17-9-11-20-18(13-17)10-12-21(24)22(20)15-5-2-1-3-6-15/h1-14,23-24H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E2 from human placental 17beta-HSD2 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50335817

(6-(3-hydroxyphenyl)-1-(6-methoxypyridin-3-yl)napht...)Show SMILES COc1ccc(cn1)-c1c(O)ccc2cc(ccc12)-c1cccc(O)c1 Show InChI InChI=1S/C22H17NO3/c1-26-21-10-7-17(13-23-21)22-19-8-5-15(11-16(19)6-9-20(22)25)14-3-2-4-18(24)12-14/h2-13,24-25H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E1 from 17beta-HSD1 in human T47D cells after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50335819

(1-(3-Aminophenyl)-6-(3-hydroxyphenyl)-2-naphthol |...)Show SMILES Nc1cccc(c1)-c1c(O)ccc2cc(ccc12)-c1cccc(O)c1 Show InChI InChI=1S/C22H17NO2/c23-18-5-1-4-17(12-18)22-20-9-7-15(11-16(20)8-10-21(22)25)14-3-2-6-19(24)13-14/h1-13,24-25H,23H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50335818

(6-(3-Hydroxyphenyl)-1-(pyridin-3-yl)-2-naphthol | ...)Show InChI InChI=1S/C21H15NO2/c23-18-5-1-3-14(12-18)15-6-8-19-16(11-15)7-9-20(24)21(19)17-4-2-10-22-13-17/h1-13,23-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50335816

(1,6-Bis(3-hydroxyphenyl)-2-naphthol | CHEMBL165065...)Show SMILES Oc1cccc(c1)-c1ccc2c(c(O)ccc2c1)-c1cccc(O)c1 Show InChI InChI=1S/C22H16O3/c23-18-5-1-3-14(12-18)15-7-9-20-16(11-15)8-10-21(25)22(20)17-4-2-6-19(24)13-17/h1-13,23-25H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 959 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E2 from human placental 17beta-HSD2 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50335819

(1-(3-Aminophenyl)-6-(3-hydroxyphenyl)-2-naphthol |...)Show SMILES Nc1cccc(c1)-c1c(O)ccc2cc(ccc12)-c1cccc(O)c1 Show InChI InChI=1S/C22H17NO2/c23-18-5-1-4-17(12-18)22-20-9-7-15(11-16(20)8-10-21(22)25)14-3-2-6-19(24)13-14/h1-13,24-25H,23H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50335818

(6-(3-Hydroxyphenyl)-1-(pyridin-3-yl)-2-naphthol | ...)Show InChI InChI=1S/C21H15NO2/c23-18-5-1-3-14(12-18)15-6-8-19-16(11-15)7-9-20(24)21(19)17-4-2-10-22-13-17/h1-13,23-24H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E2 from human placental 17beta-HSD2 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50335819

(1-(3-Aminophenyl)-6-(3-hydroxyphenyl)-2-naphthol |...)Show SMILES Nc1cccc(c1)-c1c(O)ccc2cc(ccc12)-c1cccc(O)c1 Show InChI InChI=1S/C22H17NO2/c23-18-5-1-4-17(12-18)22-20-9-7-15(11-16(20)8-10-21(22)25)14-3-2-6-19(24)13-14/h1-13,24-25H,23H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50261942

(6-(3-Hydroxyphenyl)-1-phenyl-2-naphthol | CHEMBL46...)Show InChI InChI=1S/C22H16O2/c23-19-8-4-7-16(14-19)17-9-11-20-18(13-17)10-12-21(24)22(20)15-5-2-1-3-6-15/h1-14,23-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50261942

(6-(3-Hydroxyphenyl)-1-phenyl-2-naphthol | CHEMBL46...)Show InChI InChI=1S/C22H16O2/c23-19-8-4-7-16(14-19)17-9-11-20-18(13-17)10-12-21(24)22(20)15-5-2-1-3-6-15/h1-14,23-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50335819

(1-(3-Aminophenyl)-6-(3-hydroxyphenyl)-2-naphthol |...)Show SMILES Nc1cccc(c1)-c1c(O)ccc2cc(ccc12)-c1cccc(O)c1 Show InChI InChI=1S/C22H17NO2/c23-18-5-1-4-17(12-18)22-20-9-7-15(11-16(20)8-10-21(22)25)14-3-2-6-19(24)13-14/h1-13,24-25H,23H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E2 from human placental 17beta-HSD2 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50261942

(6-(3-Hydroxyphenyl)-1-phenyl-2-naphthol | CHEMBL46...)Show InChI InChI=1S/C22H16O2/c23-19-8-4-7-16(14-19)17-9-11-20-18(13-17)10-12-21(24)22(20)15-5-2-1-3-6-15/h1-14,23-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50240772

((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50236897
(3-(furan-2-ylmethyl)-1,8-dimethyl-1H-purine-2,6(3H...)Show InChI InChI=1S/C12H12N4O3/c1-7-13-9-10(14-7)16(6-8-4-3-5-19-8)12(18)15(2)11(9)17/h3-5H,6H2,1-2H3,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50335820

(6-(3-Hydroxyphenyl)-1-(pyridin-4-yl)-2-naphthol | ...)Show InChI InChI=1S/C21H15NO2/c23-18-3-1-2-15(13-18)16-4-6-19-17(12-16)5-7-20(24)21(19)14-8-10-22-11-9-14/h1-13,23-24H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E2 from human placental 17beta-HSD2 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50335818

(6-(3-Hydroxyphenyl)-1-(pyridin-3-yl)-2-naphthol | ...)Show InChI InChI=1S/C21H15NO2/c23-18-5-1-3-14(12-18)15-6-8-19-16(11-15)7-9-20(24)21(19)17-4-2-10-22-13-17/h1-13,23-24H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2B6 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50168371

(6-(3-Hydroxy-phenyl)-naphthalen-2-ol | 6-(3-hydrox...)Show InChI InChI=1S/C16H12O2/c17-15-3-1-2-11(9-15)12-4-5-14-10-16(18)7-6-13(14)8-12/h1-10,17-18H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Displacement of [3H]E2 from human placental 17beta-HSD2 after 10 mins by fluid scintillation counting |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50335816

(1,6-Bis(3-hydroxyphenyl)-2-naphthol | CHEMBL165065...)Show SMILES Oc1cccc(c1)-c1ccc2c(c(O)ccc2c1)-c1cccc(O)c1 Show InChI InChI=1S/C22H16O3/c23-18-5-1-3-14(12-18)15-7-9-20-16(11-15)8-10-21(25)22(20)17-4-2-6-19(24)13-17/h1-13,23-25H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50240772

((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2/t8-,9+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2B6 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50261942

(6-(3-Hydroxyphenyl)-1-phenyl-2-naphthol | CHEMBL46...)Show InChI InChI=1S/C22H16O2/c23-19-8-4-7-16(14-19)17-9-11-20-18(13-17)10-12-21(24)22(20)15-5-2-1-3-6-15/h1-14,23-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50261942

(6-(3-Hydroxyphenyl)-1-phenyl-2-naphthol | CHEMBL46...)Show InChI InChI=1S/C22H16O2/c23-19-8-4-7-16(14-19)17-9-11-20-18(13-17)10-12-21(24)22(20)15-5-2-1-3-6-15/h1-14,23-24H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2B6 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50335819

(1-(3-Aminophenyl)-6-(3-hydroxyphenyl)-2-naphthol |...)Show SMILES Nc1cccc(c1)-c1c(O)ccc2cc(ccc12)-c1cccc(O)c1 Show InChI InChI=1S/C22H17NO2/c23-18-5-1-4-17(12-18)22-20-9-7-15(11-16(20)8-10-21(22)25)14-3-2-6-19(24)13-14/h1-13,24-25H,23H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2B6 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50335819

(1-(3-Aminophenyl)-6-(3-hydroxyphenyl)-2-naphthol |...)Show SMILES Nc1cccc(c1)-c1c(O)ccc2cc(ccc12)-c1cccc(O)c1 Show InChI InChI=1S/C22H17NO2/c23-18-5-1-4-17(12-18)22-20-9-7-15(11-16(20)8-10-21(22)25)14-3-2-6-19(24)13-14/h1-13,24-25H,23H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50335816

(1,6-Bis(3-hydroxyphenyl)-2-naphthol | CHEMBL165065...)Show SMILES Oc1cccc(c1)-c1ccc2c(c(O)ccc2c1)-c1cccc(O)c1 Show InChI InChI=1S/C22H16O3/c23-18-5-1-3-14(12-18)15-7-9-20-16(11-15)8-10-21(25)22(20)17-4-2-6-19(24)13-17/h1-13,23-25H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2B6 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50261942

(6-(3-Hydroxyphenyl)-1-phenyl-2-naphthol | CHEMBL46...)Show InChI InChI=1S/C22H16O2/c23-19-8-4-7-16(14-19)17-9-11-20-18(13-17)10-12-21(24)22(20)15-5-2-1-3-6-15/h1-14,23-24H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50335816

(1,6-Bis(3-hydroxyphenyl)-2-naphthol | CHEMBL165065...)Show SMILES Oc1cccc(c1)-c1ccc2c(c(O)ccc2c1)-c1cccc(O)c1 Show InChI InChI=1S/C22H16O3/c23-18-5-1-3-14(12-18)15-7-9-20-16(11-15)8-10-21(25)22(20)17-4-2-6-19(24)13-17/h1-13,23-25H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50335818

(6-(3-Hydroxyphenyl)-1-(pyridin-3-yl)-2-naphthol | ...)Show InChI InChI=1S/C21H15NO2/c23-18-5-1-3-14(12-18)15-6-8-19-16(11-15)7-9-20(24)21(19)17-4-2-10-22-13-17/h1-13,23-24H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50335816

(1,6-Bis(3-hydroxyphenyl)-2-naphthol | CHEMBL165065...)Show SMILES Oc1cccc(c1)-c1ccc2c(c(O)ccc2c1)-c1cccc(O)c1 Show InChI InChI=1S/C22H16O3/c23-18-5-1-3-14(12-18)15-7-9-20-16(11-15)8-10-21(25)22(20)17-4-2-6-19(24)13-17/h1-13,23-25H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50335819

(1-(3-Aminophenyl)-6-(3-hydroxyphenyl)-2-naphthol |...)Show SMILES Nc1cccc(c1)-c1c(O)ccc2cc(ccc12)-c1cccc(O)c1 Show InChI InChI=1S/C22H17NO2/c23-18-5-1-4-17(12-18)22-20-9-7-15(11-16(20)8-10-21(22)25)14-3-2-6-19(24)13-14/h1-13,24-25H,23H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data