 Found 75 hits of Enzyme Inhibition Constant Data
Found 75 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297308
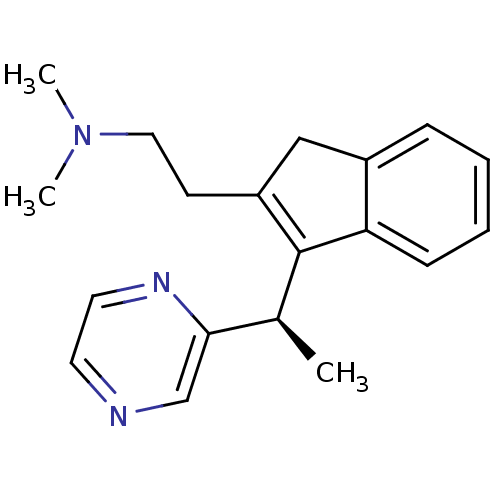
((-)-Dimethyl-{2-[3-((R)-1-pyrazin-2-yl-ethyl)-1H-i...)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C19H23N3/c1-14(18-13-20-9-10-21-18)19-16(8-11-22(2)3)12-15-6-4-5-7-17(15)19/h4-7,9-10,13-14H,8,11-12H2,1-3H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336074
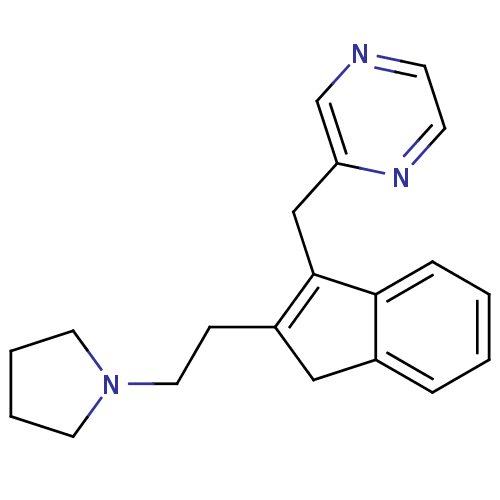
(2-((2-(2-(pyrrolidin-1-yl)ethyl)-1H-inden-3-yl)met...)Show InChI InChI=1S/C20H23N3/c1-2-6-19-16(5-1)13-17(7-12-23-10-3-4-11-23)20(19)14-18-15-21-8-9-22-18/h1-2,5-6,8-9,15H,3-4,7,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297305
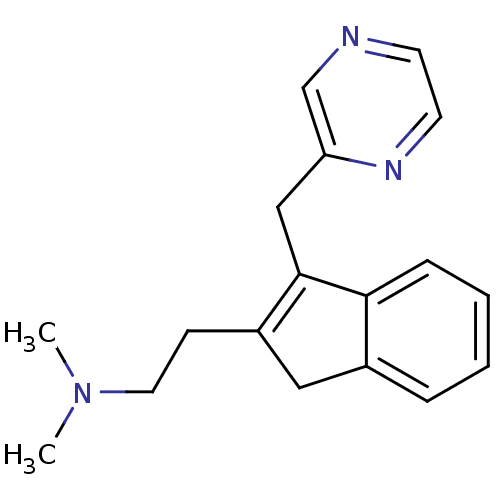
(CHEMBL559251 | Dimethyl-[2-(3-pyrazin-2ylmethyl-1H...)Show InChI InChI=1S/C18H21N3/c1-21(2)10-7-15-11-14-5-3-4-6-17(14)18(15)12-16-13-19-8-9-20-16/h3-6,8-9,13H,7,10-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336073

(2-((2-(2-(azetidin-1-yl)ethyl)-1H-inden-3-yl)methy...)Show InChI InChI=1S/C19H21N3/c1-2-5-18-15(4-1)12-16(6-11-22-9-3-10-22)19(18)13-17-14-20-7-8-21-17/h1-2,4-5,7-8,14H,3,6,9-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336086
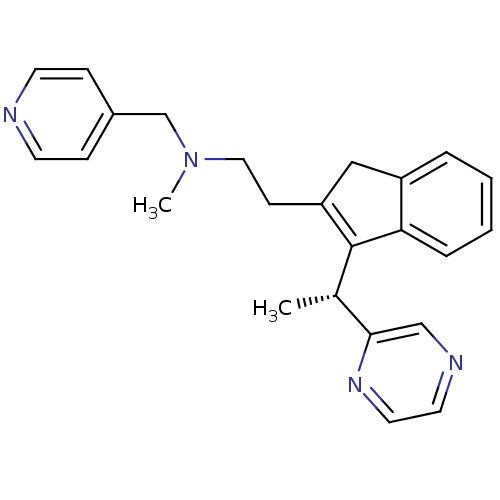
((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)Cc2ccncc2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C24H26N4/c1-18(23-16-26-12-13-27-23)24-21(15-20-5-3-4-6-22(20)24)9-14-28(2)17-19-7-10-25-11-8-19/h3-8,10-13,16,18H,9,14-15,17H2,1-2H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336080

((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)Cc2cccs2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C23H25N3S/c1-17(22-15-24-10-11-25-22)23-19(14-18-6-3-4-8-21(18)23)9-12-26(2)16-20-7-5-13-27-20/h3-8,10-11,13,15,17H,9,12,14,16H2,1-2H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336066

(CHEMBL1669405 | N-ethyl-N-methyl-2-(3-(pyrazin-2-y...)Show InChI InChI=1S/C19H23N3/c1-3-22(2)11-8-16-12-15-6-4-5-7-18(15)19(16)13-17-14-20-9-10-21-17/h4-7,9-10,14H,3,8,11-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336079
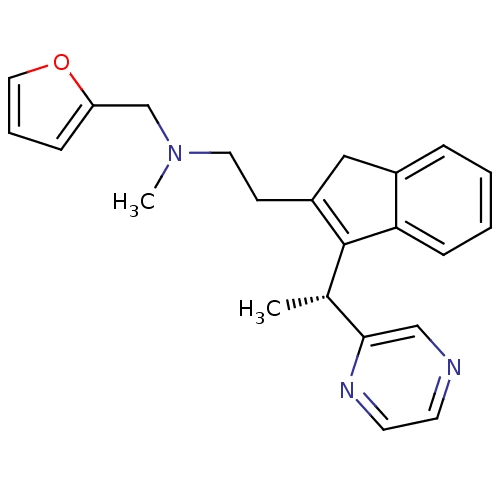
((R)-N-(furan-2-ylmethyl)-N-methyl-2-(3-(1-(pyrazin...)Show SMILES C[C@H](C1=C(CCN(C)Cc2ccco2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C23H25N3O/c1-17(22-15-24-10-11-25-22)23-19(14-18-6-3-4-8-21(18)23)9-12-26(2)16-20-7-5-13-27-20/h3-8,10-11,13,15,17H,9,12,14,16H2,1-2H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336083
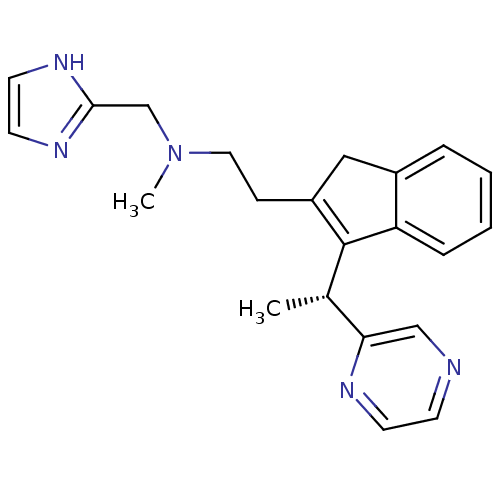
((R)-N-((1H-imidazol-2-yl)methyl)-N-methyl-2-(3-(1-...)Show SMILES C[C@H](C1=C(CCN(C)Cc2ncc[nH]2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C22H25N5/c1-16(20-14-23-8-9-24-20)22-18(13-17-5-3-4-6-19(17)22)7-12-27(2)15-21-25-10-11-26-21/h3-6,8-11,14,16H,7,12-13,15H2,1-2H3,(H,25,26)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336078

(2-((2-(2-(3-fluoropyrrolidin-1-yl)ethyl)-1H-inden-...)Show SMILES FC1CCN(CCC2=C(Cc3cnccn3)c3ccccc3C2)C1 |c:7| Show InChI InChI=1S/C20H22FN3/c21-17-6-10-24(14-17)9-5-16-11-15-3-1-2-4-19(15)20(16)12-18-13-22-7-8-23-18/h1-4,7-8,13,17H,5-6,9-12,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336069

((R)-2-fluoro-N-methyl-N-(2-(3-(1-(pyrazin-2-yl)eth...)Show SMILES C[C@H](C1=C(CCN(C)CCF)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C20H24FN3/c1-15(19-14-22-9-10-23-19)20-17(7-11-24(2)12-8-21)13-16-5-3-4-6-18(16)20/h3-6,9-10,14-15H,7-8,11-13H2,1-2H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336085

((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)Cc2ccccn2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C24H26N4/c1-18(23-16-25-12-13-27-23)24-20(15-19-7-3-4-9-22(19)24)10-14-28(2)17-21-8-5-6-11-26-21/h3-9,11-13,16,18H,10,14-15,17H2,1-2H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336088

((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)CC2CCOCC2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C24H31N3O/c1-18(23-16-25-10-11-26-23)24-21(15-20-5-3-4-6-22(20)24)7-12-27(2)17-19-8-13-28-14-9-19/h3-6,10-11,16,18-19H,7-9,12-15,17H2,1-2H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336068

(2-fluoro-N-methyl-N-(2-(3-(pyrazin-2-ylmethyl)-1H-...)Show InChI InChI=1S/C19H22FN3/c1-23(11-7-20)10-6-16-12-15-4-2-3-5-18(15)19(16)13-17-14-21-8-9-22-17/h2-5,8-9,14H,6-7,10-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336087
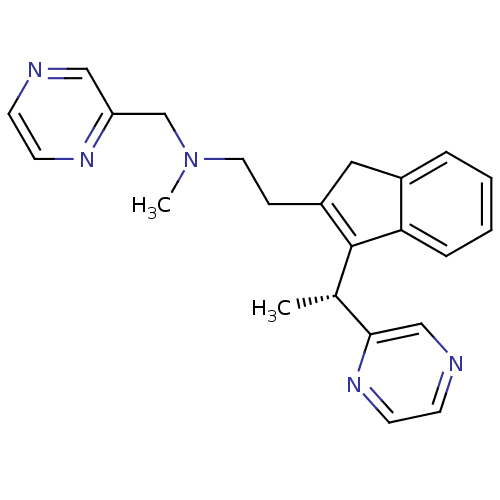
((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)Cc2cnccn2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C23H25N5/c1-17(22-15-25-9-11-27-22)23-19(13-18-5-3-4-6-21(18)23)7-12-28(2)16-20-14-24-8-10-26-20/h3-6,8-11,14-15,17H,7,12-13,16H2,1-2H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336084

((R)-N-methyl-N-((1-methyl-1H-imidazol-2-yl)methyl)...)Show SMILES C[C@H](C1=C(CCN(C)Cc2nccn2C)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C23H27N5/c1-17(21-15-24-9-10-25-21)23-19(14-18-6-4-5-7-20(18)23)8-12-27(2)16-22-26-11-13-28(22)3/h4-7,9-11,13,15,17H,8,12,14,16H2,1-3H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336070

((R)-2-(methyl(2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inde...)Show SMILES C[C@H](C1=C(CCN(C)CC#N)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C20H22N4/c1-15(19-14-22-9-10-23-19)20-17(7-11-24(2)12-8-21)13-16-5-3-4-6-18(16)20/h3-6,9-10,14-15H,7,11-13H2,1-2H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336067

(CHEMBL1669406 | N-methyl-N-(2-(3-(pyrazin-2-ylmeth...)Show InChI InChI=1S/C20H25N3/c1-3-11-23(2)12-8-17-13-16-6-4-5-7-19(16)20(17)14-18-15-21-9-10-22-18/h4-7,9-10,15H,3,8,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336081

((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)Cc2nccs2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C22H24N4S/c1-16(20-14-23-8-9-24-20)22-18(13-17-5-3-4-6-19(17)22)7-11-26(2)15-21-25-10-12-27-21/h3-6,8-10,12,14,16H,7,11,13,15H2,1-2H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336075

(2-((2-(2-(3-fluoroazetidin-1-yl)ethyl)-1H-inden-3-...)Show InChI InChI=1S/C19H20FN3/c20-16-12-23(13-16)8-5-15-9-14-3-1-2-4-18(14)19(15)10-17-11-21-6-7-22-17/h1-4,6-7,11,16H,5,8-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336072
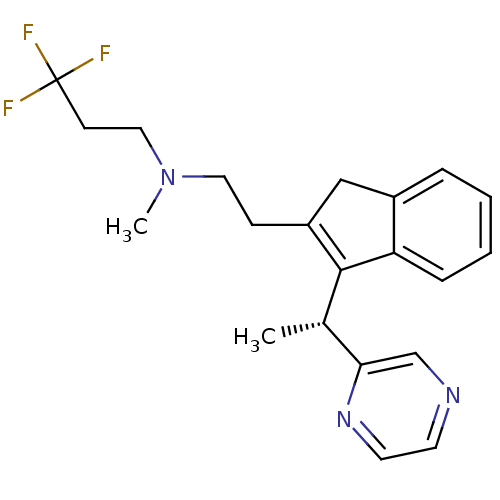
((R)-3,3,3-trifluoro-N-methyl-N-(2-(3-(1-(pyrazin-2...)Show SMILES C[C@H](C1=C(CCN(C)CCC(F)(F)F)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C21H24F3N3/c1-15(19-14-25-9-10-26-19)20-17(13-16-5-3-4-6-18(16)20)7-11-27(2)12-8-21(22,23)24/h3-6,9-10,14-15H,7-8,11-13H2,1-2H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336071

((R)-3-(methyl(2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inde...)Show SMILES C[C@H](C1=C(CCN(C)CCC#N)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C21H24N4/c1-16(20-15-23-10-11-24-20)21-18(8-13-25(2)12-5-9-22)14-17-6-3-4-7-19(17)21/h3-4,6-7,10-11,15-16H,5,8,12-14H2,1-2H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336082
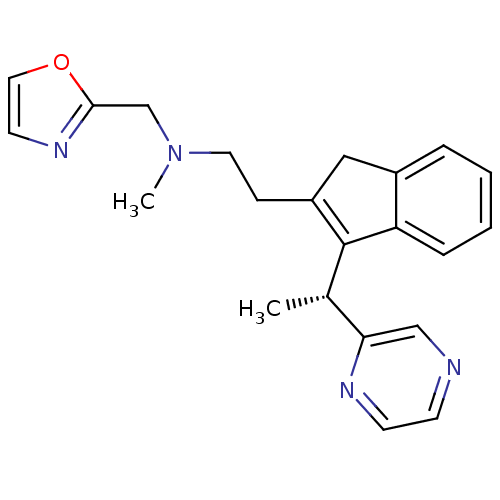
((R)-N-methyl-N-(oxazol-2-ylmethyl)-2-(3-(1-(pyrazi...)Show SMILES C[C@H](C1=C(CCN(C)Cc2ncco2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C22H24N4O/c1-16(20-14-23-8-9-24-20)22-18(13-17-5-3-4-6-19(17)22)7-11-26(2)15-21-25-10-12-27-21/h3-6,8-10,12,14,16H,7,11,13,15H2,1-2H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336077
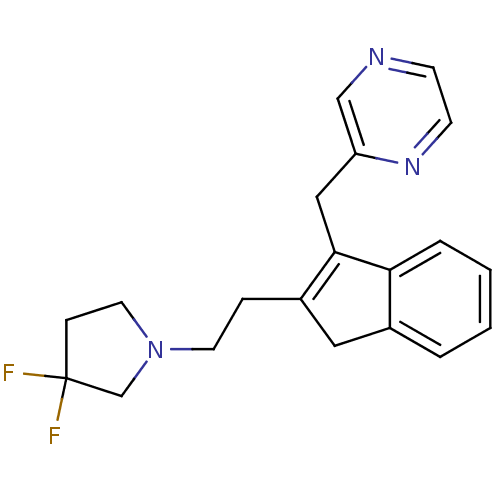
(2-((2-(2-(3,3-difluoropyrrolidin-1-yl)ethyl)-1H-in...)Show SMILES FC1(F)CCN(CCC2=C(Cc3cnccn3)c3ccccc3C2)C1 |c:8| Show InChI InChI=1S/C20H21F2N3/c21-20(22)6-10-25(14-20)9-5-16-11-15-3-1-2-4-18(15)19(16)12-17-13-23-7-8-24-17/h1-4,7-8,13H,5-6,9-12,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 249 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336076

(2-((2-(2-(3,3-difluoroazetidin-1-yl)ethyl)-1H-inde...)Show SMILES FC1(F)CN(CCC2=C(Cc3cnccn3)c3ccccc3C2)C1 |c:7| Show InChI InChI=1S/C19H19F2N3/c20-19(21)12-24(13-19)8-5-15-9-14-3-1-2-4-17(14)18(15)10-16-11-22-6-7-23-16/h1-4,6-7,11H,5,8-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336067

(CHEMBL1669406 | N-methyl-N-(2-(3-(pyrazin-2-ylmeth...)Show InChI InChI=1S/C20H25N3/c1-3-11-23(2)12-8-17-13-16-6-4-5-7-19(16)20(17)14-18-15-21-9-10-22-18/h4-7,9-10,15H,3,8,11-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 973 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336086

((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)Cc2ccncc2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C24H26N4/c1-18(23-16-26-12-13-27-23)24-21(15-20-5-3-4-6-22(20)24)9-14-28(2)17-19-7-10-25-11-8-19/h3-8,10-13,16,18H,9,14-15,17H2,1-2H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336077

(2-((2-(2-(3,3-difluoropyrrolidin-1-yl)ethyl)-1H-in...)Show SMILES FC1(F)CCN(CCC2=C(Cc3cnccn3)c3ccccc3C2)C1 |c:8| Show InChI InChI=1S/C20H21F2N3/c21-20(22)6-10-25(14-20)9-5-16-11-15-3-1-2-4-18(15)19(16)12-17-13-23-7-8-24-17/h1-4,7-8,13H,5-6,9-12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336080

((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)Cc2cccs2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C23H25N3S/c1-17(22-15-24-10-11-25-22)23-19(14-18-6-3-4-8-21(18)23)9-12-26(2)16-20-7-5-13-27-20/h3-8,10-11,13,15,17H,9,12,14,16H2,1-2H3/t17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336084

((R)-N-methyl-N-((1-methyl-1H-imidazol-2-yl)methyl)...)Show SMILES C[C@H](C1=C(CCN(C)Cc2nccn2C)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C23H27N5/c1-17(21-15-24-9-10-25-21)23-19(14-18-6-4-5-7-20(18)23)8-12-27(2)16-22-26-11-13-28(22)3/h4-7,9-11,13,15,17H,8,12,14,16H2,1-3H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336080

((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)Cc2cccs2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C23H25N3S/c1-17(22-15-24-10-11-25-22)23-19(14-18-6-3-4-8-21(18)23)9-12-26(2)16-20-7-5-13-27-20/h3-8,10-11,13,15,17H,9,12,14,16H2,1-2H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336069

((R)-2-fluoro-N-methyl-N-(2-(3-(1-(pyrazin-2-yl)eth...)Show SMILES C[C@H](C1=C(CCN(C)CCF)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C20H24FN3/c1-15(19-14-22-9-10-23-19)20-17(7-11-24(2)12-8-21)13-16-5-3-4-6-18(16)20/h3-6,9-10,14-15H,7-8,11-13H2,1-2H3/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336079

((R)-N-(furan-2-ylmethyl)-N-methyl-2-(3-(1-(pyrazin...)Show SMILES C[C@H](C1=C(CCN(C)Cc2ccco2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C23H25N3O/c1-17(22-15-24-10-11-25-22)23-19(14-18-6-3-4-8-21(18)23)9-12-26(2)16-20-7-5-13-27-20/h3-8,10-11,13,15,17H,9,12,14,16H2,1-2H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336081

((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)Cc2nccs2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C22H24N4S/c1-16(20-14-23-8-9-24-20)22-18(13-17-5-3-4-6-19(17)22)7-11-26(2)15-21-25-10-12-27-21/h3-6,8-10,12,14,16H,7,11,13,15H2,1-2H3/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336068

(2-fluoro-N-methyl-N-(2-(3-(pyrazin-2-ylmethyl)-1H-...)Show InChI InChI=1S/C19H22FN3/c1-23(11-7-20)10-6-16-12-15-4-2-3-5-18(15)19(16)13-17-14-21-8-9-22-17/h2-5,8-9,14H,6-7,10-13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336074

(2-((2-(2-(pyrrolidin-1-yl)ethyl)-1H-inden-3-yl)met...)Show InChI InChI=1S/C20H23N3/c1-2-6-19-16(5-1)13-17(7-12-23-10-3-4-11-23)20(19)14-18-15-21-8-9-22-18/h1-2,5-6,8-9,15H,3-4,7,10-14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336076

(2-((2-(2-(3,3-difluoroazetidin-1-yl)ethyl)-1H-inde...)Show SMILES FC1(F)CN(CCC2=C(Cc3cnccn3)c3ccccc3C2)C1 |c:7| Show InChI InChI=1S/C19H19F2N3/c20-19(21)12-24(13-19)8-5-15-9-14-3-1-2-4-17(14)18(15)10-16-11-22-6-7-23-16/h1-4,6-7,11H,5,8-10,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336067

(CHEMBL1669406 | N-methyl-N-(2-(3-(pyrazin-2-ylmeth...)Show InChI InChI=1S/C20H25N3/c1-3-11-23(2)12-8-17-13-16-6-4-5-7-19(16)20(17)14-18-15-21-9-10-22-18/h4-7,9-10,15H,3,8,11-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336078

(2-((2-(2-(3-fluoropyrrolidin-1-yl)ethyl)-1H-inden-...)Show SMILES FC1CCN(CCC2=C(Cc3cnccn3)c3ccccc3C2)C1 |c:7| Show InChI InChI=1S/C20H22FN3/c21-17-6-10-24(14-17)9-5-16-11-15-3-1-2-4-19(15)20(16)12-18-13-22-7-8-23-18/h1-4,7-8,13,17H,5-6,9-12,14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336066

(CHEMBL1669405 | N-ethyl-N-methyl-2-(3-(pyrazin-2-y...)Show InChI InChI=1S/C19H23N3/c1-3-22(2)11-8-16-12-15-6-4-5-7-18(15)19(16)13-17-14-20-9-10-21-17/h4-7,9-10,14H,3,8,11-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336079

((R)-N-(furan-2-ylmethyl)-N-methyl-2-(3-(1-(pyrazin...)Show SMILES C[C@H](C1=C(CCN(C)Cc2ccco2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C23H25N3O/c1-17(22-15-24-10-11-25-22)23-19(14-18-6-3-4-8-21(18)23)9-12-26(2)16-20-7-5-13-27-20/h3-8,10-11,13,15,17H,9,12,14,16H2,1-2H3/t17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336083

((R)-N-((1H-imidazol-2-yl)methyl)-N-methyl-2-(3-(1-...)Show SMILES C[C@H](C1=C(CCN(C)Cc2ncc[nH]2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C22H25N5/c1-16(20-14-23-8-9-24-20)22-18(13-17-5-3-4-6-19(17)22)7-12-27(2)15-21-25-10-11-26-21/h3-6,8-11,14,16H,7,12-13,15H2,1-2H3,(H,25,26)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336085

((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)Cc2ccccn2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C24H26N4/c1-18(23-16-25-12-13-27-23)24-20(15-19-7-3-4-9-22(19)24)10-14-28(2)17-21-8-5-6-11-26-21/h3-9,11-13,16,18H,10,14-15,17H2,1-2H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336082

((R)-N-methyl-N-(oxazol-2-ylmethyl)-2-(3-(1-(pyrazi...)Show SMILES C[C@H](C1=C(CCN(C)Cc2ncco2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C22H24N4O/c1-16(20-14-23-8-9-24-20)22-18(13-17-5-3-4-6-19(17)22)7-11-26(2)15-21-25-10-12-27-21/h3-6,8-10,12,14,16H,7,11,13,15H2,1-2H3/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336072

((R)-3,3,3-trifluoro-N-methyl-N-(2-(3-(1-(pyrazin-2...)Show SMILES C[C@H](C1=C(CCN(C)CCC(F)(F)F)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C21H24F3N3/c1-15(19-14-25-9-10-26-19)20-17(13-16-5-3-4-6-18(16)20)7-11-27(2)12-8-21(22,23)24/h3-6,9-10,14-15H,7-8,11-13H2,1-2H3/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336084

((R)-N-methyl-N-((1-methyl-1H-imidazol-2-yl)methyl)...)Show SMILES C[C@H](C1=C(CCN(C)Cc2nccn2C)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C23H27N5/c1-17(21-15-24-9-10-25-21)23-19(14-18-6-4-5-7-20(18)23)8-12-27(2)16-22-26-11-13-28(22)3/h4-7,9-11,13,15,17H,8,12,14,16H2,1-3H3/t17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336085

((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)Cc2ccccn2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C24H26N4/c1-18(23-16-25-12-13-27-23)24-20(15-19-7-3-4-9-22(19)24)10-14-28(2)17-21-8-5-6-11-26-21/h3-9,11-13,16,18H,10,14-15,17H2,1-2H3/t18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336083

((R)-N-((1H-imidazol-2-yl)methyl)-N-methyl-2-(3-(1-...)Show SMILES C[C@H](C1=C(CCN(C)Cc2ncc[nH]2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C22H25N5/c1-16(20-14-23-8-9-24-20)22-18(13-17-5-3-4-6-19(17)22)7-12-27(2)15-21-25-10-11-26-21/h3-6,8-11,14,16H,7,12-13,15H2,1-2H3,(H,25,26)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336075

(2-((2-(2-(3-fluoroazetidin-1-yl)ethyl)-1H-inden-3-...)Show InChI InChI=1S/C19H20FN3/c20-16-12-23(13-16)8-5-15-9-14-3-1-2-4-18(14)19(15)10-17-11-21-6-7-22-17/h1-4,6-7,11,16H,5,8-10,12-13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297305

(CHEMBL559251 | Dimethyl-[2-(3-pyrazin-2ylmethyl-1H...)Show InChI InChI=1S/C18H21N3/c1-21(2)10-7-15-11-14-5-3-4-6-17(14)18(15)12-16-13-19-8-9-20-16/h3-6,8-9,13H,7,10-12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336072

((R)-3,3,3-trifluoro-N-methyl-N-(2-(3-(1-(pyrazin-2...)Show SMILES C[C@H](C1=C(CCN(C)CCC(F)(F)F)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C21H24F3N3/c1-15(19-14-25-9-10-26-19)20-17(13-16-5-3-4-6-18(16)20)7-11-27(2)12-8-21(22,23)24/h3-6,9-10,14-15H,7-8,11-13H2,1-2H3/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336077

(2-((2-(2-(3,3-difluoropyrrolidin-1-yl)ethyl)-1H-in...)Show SMILES FC1(F)CCN(CCC2=C(Cc3cnccn3)c3ccccc3C2)C1 |c:8| Show InChI InChI=1S/C20H21F2N3/c21-20(22)6-10-25(14-20)9-5-16-11-15-3-1-2-4-18(15)19(16)12-17-13-23-7-8-24-17/h1-4,7-8,13H,5-6,9-12,14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336086

((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)Cc2ccncc2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C24H26N4/c1-18(23-16-26-12-13-27-23)24-21(15-20-5-3-4-6-22(20)24)9-14-28(2)17-19-7-10-25-11-8-19/h3-8,10-13,16,18H,9,14-15,17H2,1-2H3/t18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336073

(2-((2-(2-(azetidin-1-yl)ethyl)-1H-inden-3-yl)methy...)Show InChI InChI=1S/C19H21N3/c1-2-5-18-15(4-1)12-16(6-11-22-9-3-10-22)19(18)13-17-14-20-7-8-21-17/h1-2,4-5,7-8,14H,3,6,9-13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336088

((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)CC2CCOCC2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C24H31N3O/c1-18(23-16-25-10-11-26-23)24-21(15-20-5-3-4-6-22(20)24)7-12-27(2)17-19-8-13-28-14-9-19/h3-6,10-11,16,18-19H,7-9,12-15,17H2,1-2H3/t18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336074

(2-((2-(2-(pyrrolidin-1-yl)ethyl)-1H-inden-3-yl)met...)Show InChI InChI=1S/C20H23N3/c1-2-6-19-16(5-1)13-17(7-12-23-10-3-4-11-23)20(19)14-18-15-21-8-9-22-18/h1-2,5-6,8-9,15H,3-4,7,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336070

((R)-2-(methyl(2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inde...)Show SMILES C[C@H](C1=C(CCN(C)CC#N)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C20H22N4/c1-15(19-14-22-9-10-23-19)20-17(7-11-24(2)12-8-21)13-16-5-3-4-6-18(16)20/h3-6,9-10,14-15H,7,11-13H2,1-2H3/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336071

((R)-3-(methyl(2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inde...)Show SMILES C[C@H](C1=C(CCN(C)CCC#N)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C21H24N4/c1-16(20-15-23-10-11-24-20)21-18(8-13-25(2)12-5-9-22)14-17-6-3-4-7-19(17)21/h3-4,6-7,10-11,15-16H,5,8,12-14H2,1-2H3/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50297305

(CHEMBL559251 | Dimethyl-[2-(3-pyrazin-2ylmethyl-1H...)Show InChI InChI=1S/C18H21N3/c1-21(2)10-7-15-11-14-5-3-4-6-17(14)18(15)12-16-13-19-8-9-20-16/h3-6,8-9,13H,7,10-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50297308

((-)-Dimethyl-{2-[3-((R)-1-pyrazin-2-yl-ethyl)-1H-i...)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C19H23N3/c1-14(18-13-20-9-10-21-18)19-16(8-11-22(2)3)12-15-6-4-5-7-17(15)19/h4-7,9-10,13-14H,8,11-12H2,1-3H3/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336070

((R)-2-(methyl(2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inde...)Show SMILES C[C@H](C1=C(CCN(C)CC#N)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C20H22N4/c1-15(19-14-22-9-10-23-19)20-17(7-11-24(2)12-8-21)13-16-5-3-4-6-18(16)20/h3-6,9-10,14-15H,7,11-13H2,1-2H3/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336081

((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)Cc2nccs2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C22H24N4S/c1-16(20-14-23-8-9-24-20)22-18(13-17-5-3-4-6-19(17)22)7-11-26(2)15-21-25-10-12-27-21/h3-6,8-10,12,14,16H,7,11,13,15H2,1-2H3/t16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336088

((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)CC2CCOCC2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C24H31N3O/c1-18(23-16-25-10-11-26-23)24-21(15-20-5-3-4-6-22(20)24)7-12-27(2)17-19-8-13-28-14-9-19/h3-6,10-11,16,18-19H,7-9,12-15,17H2,1-2H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336066

(CHEMBL1669405 | N-ethyl-N-methyl-2-(3-(pyrazin-2-y...)Show InChI InChI=1S/C19H23N3/c1-3-22(2)11-8-16-12-15-6-4-5-7-18(15)19(16)13-17-14-20-9-10-21-17/h4-7,9-10,14H,3,8,11-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336087

((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)Cc2cnccn2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C23H25N5/c1-17(22-15-25-9-11-27-22)23-19(13-18-5-3-4-6-21(18)23)7-12-28(2)16-20-14-24-8-10-26-20/h3-6,8-11,14-15,17H,7,12-13,16H2,1-2H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336073

(2-((2-(2-(azetidin-1-yl)ethyl)-1H-inden-3-yl)methy...)Show InChI InChI=1S/C19H21N3/c1-2-5-18-15(4-1)12-16(6-11-22-9-3-10-22)19(18)13-17-14-20-7-8-21-17/h1-2,4-5,7-8,14H,3,6,9-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336071

((R)-3-(methyl(2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inde...)Show SMILES C[C@H](C1=C(CCN(C)CCC#N)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C21H24N4/c1-16(20-15-23-10-11-24-20)21-18(8-13-25(2)12-5-9-22)14-17-6-3-4-7-19(17)21/h3-4,6-7,10-11,15-16H,5,8,12-14H2,1-2H3/t16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297308

((-)-Dimethyl-{2-[3-((R)-1-pyrazin-2-yl-ethyl)-1H-i...)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C19H23N3/c1-14(18-13-20-9-10-21-18)19-16(8-11-22(2)3)12-15-6-4-5-7-17(15)19/h4-7,9-10,13-14H,8,11-12H2,1-3H3/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336068

(2-fluoro-N-methyl-N-(2-(3-(pyrazin-2-ylmethyl)-1H-...)Show InChI InChI=1S/C19H22FN3/c1-23(11-7-20)10-6-16-12-15-4-2-3-5-18(15)19(16)13-17-14-21-8-9-22-17/h2-5,8-9,14H,6-7,10-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336078

(2-((2-(2-(3-fluoropyrrolidin-1-yl)ethyl)-1H-inden-...)Show SMILES FC1CCN(CCC2=C(Cc3cnccn3)c3ccccc3C2)C1 |c:7| Show InChI InChI=1S/C20H22FN3/c21-17-6-10-24(14-17)9-5-16-11-15-3-1-2-4-19(15)20(16)12-18-13-22-7-8-23-18/h1-4,7-8,13,17H,5-6,9-12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336087

((R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)Cc2cnccn2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C23H25N5/c1-17(22-15-25-9-11-27-22)23-19(13-18-5-3-4-6-21(18)23)7-12-28(2)16-20-14-24-8-10-26-20/h3-6,8-11,14-15,17H,7,12-13,16H2,1-2H3/t17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336082

((R)-N-methyl-N-(oxazol-2-ylmethyl)-2-(3-(1-(pyrazi...)Show SMILES C[C@H](C1=C(CCN(C)Cc2ncco2)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C22H24N4O/c1-16(20-14-23-8-9-24-20)22-18(13-17-5-3-4-6-19(17)22)7-11-26(2)15-21-25-10-12-27-21/h3-6,8-10,12,14,16H,7,11,13,15H2,1-2H3/t16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336075

(2-((2-(2-(3-fluoroazetidin-1-yl)ethyl)-1H-inden-3-...)Show InChI InChI=1S/C19H20FN3/c20-16-12-23(13-16)8-5-15-9-14-3-1-2-4-18(14)19(15)10-17-11-21-6-7-22-17/h1-4,6-7,11,16H,5,8-10,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50336069

((R)-2-fluoro-N-methyl-N-(2-(3-(1-(pyrazin-2-yl)eth...)Show SMILES C[C@H](C1=C(CCN(C)CCF)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C20H24FN3/c1-15(19-14-22-9-10-23-19)20-17(7-11-24(2)12-8-21)13-16-5-3-4-6-18(16)20/h3-6,9-10,14-15H,7-8,11-13H2,1-2H3/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336076

(2-((2-(2-(3,3-difluoroazetidin-1-yl)ethyl)-1H-inde...)Show SMILES FC1(F)CN(CCC2=C(Cc3cnccn3)c3ccccc3C2)C1 |c:7| Show InChI InChI=1S/C19H19F2N3/c20-19(21)12-24(13-19)8-5-15-9-14-3-1-2-4-17(14)18(15)10-16-11-22-6-7-23-16/h1-4,6-7,11H,5,8-10,12-13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data