

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343856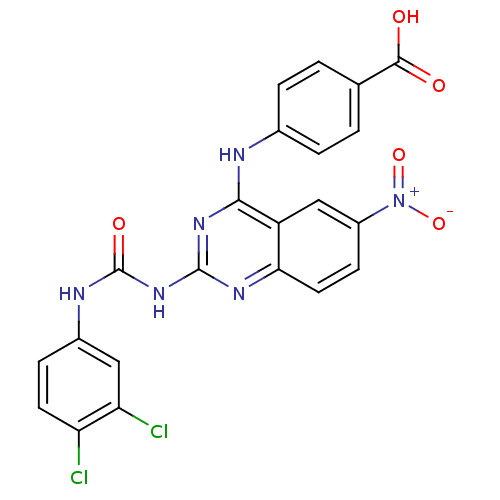 (4-(2-(3-(3,4-Dichlorophenyl)ureido)-6-nitroquinazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343862 (4-(6-Nitro-2-(4-phenoxyphenylamino)quinazolin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343858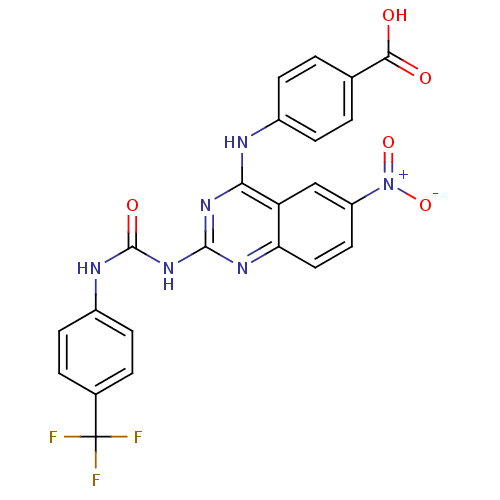 (4-(6-nitro-2-(3-(4-(trifluoromethyl)phenyl)ureido)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343857 (4-(6-nitro-2-(3-(3-(trifluoromethyl)phenyl)ureido)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343855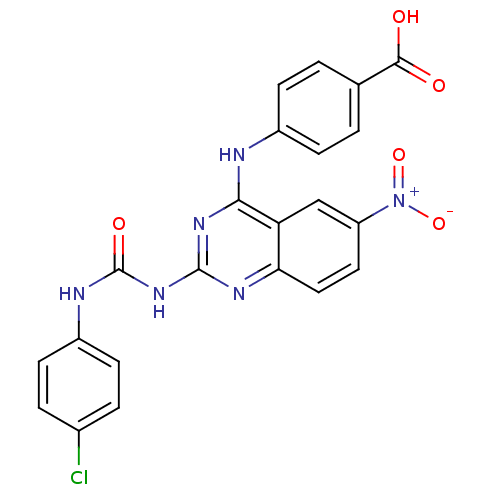 (4-(2-(3-(4-Chlorophenyl)ureido)-6-nitroquinazolin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343850 (4-(6-Nitro-4-(4-phenoxyphenylamino)quinazolin-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343852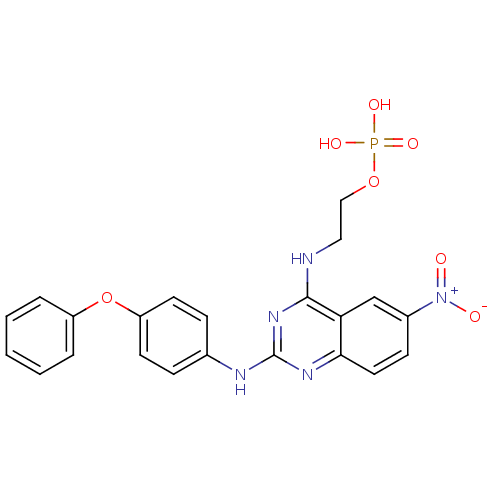 (2-(6-Nitro-2-(4-phenoxyphenylamino)quinazolin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343851 (4-(2-(4-Phenoxyphenylamino)quinazolin-4-ylamino)be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343854 (4-(2-(3-(3-Fluorophenyl)ureido)-6-nitroquinazolin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343866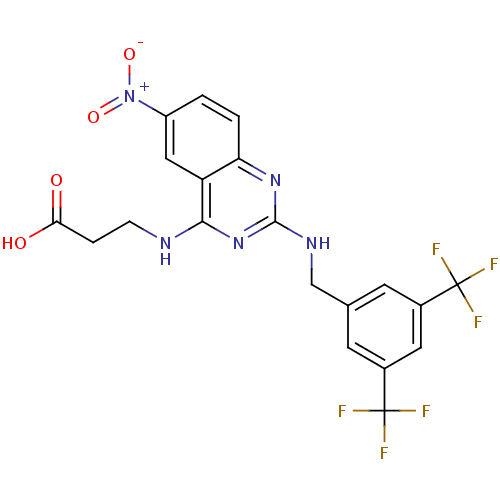 (3-(2-(3,5-Bis(trifluoromethyl)benzylamino)-6-nitro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343864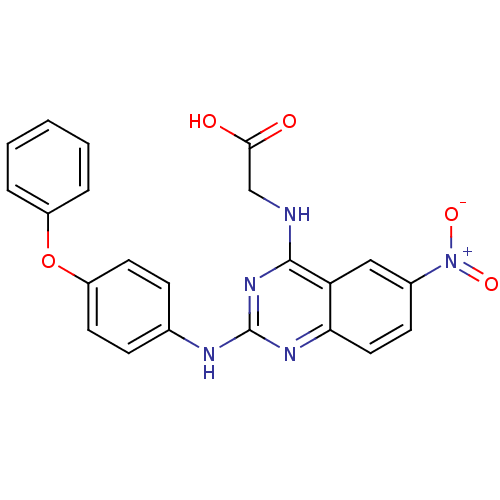 (2-(6-Nitro-2-(4-phenoxyphenylamino)quinazolin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343863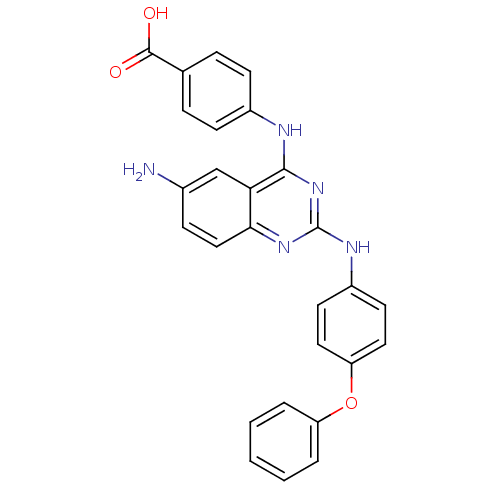 (4-(6-Amino-2-(4-phenoxyphenylamino)quinazolin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343865 (3-(6-Nitro-2-(4-phenoxyphenylamino)quinazolin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343867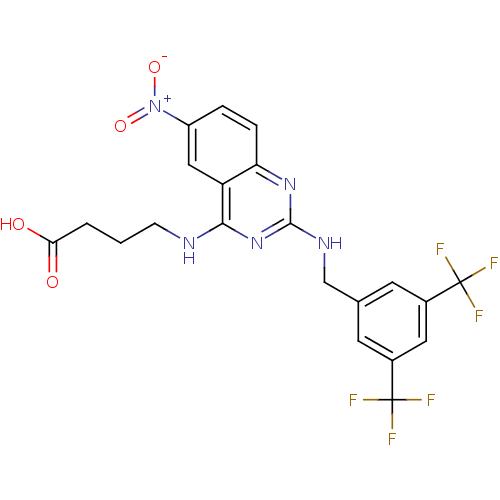 (4-(2-(3,5-Bis(trifluoromethyl)benzylamino)-6-nitro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343853 (2-(2-(Benzylamino)-6-nitroquinazolin-4-ylamino)eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343859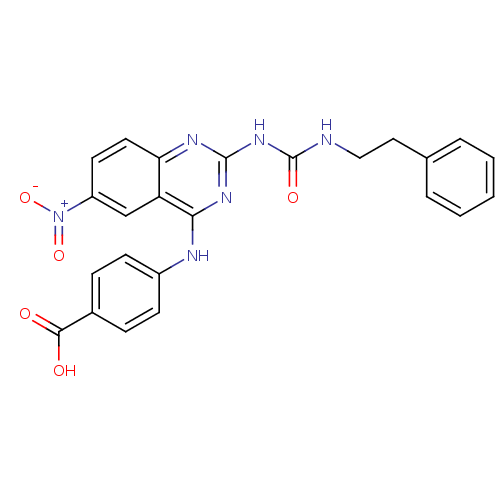 (4-(6-Nitro-2-(3-phenethylureido)quinazolin-4-ylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343860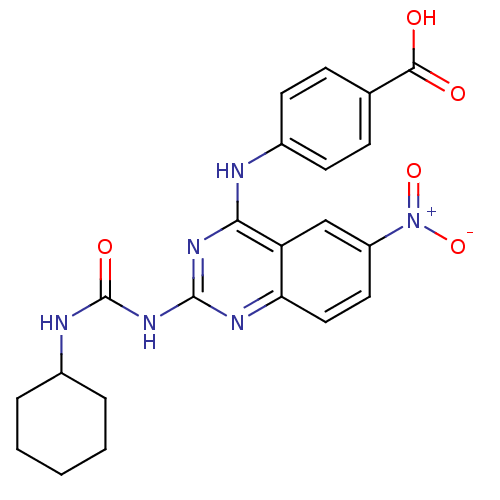 (4-(2-(3-Cyclohexylureido)-6-nitroquinazolin-4-ylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50343861 (3-(2-(3-(3,4-dichlorophenyl)ureido)-6-nitroquinazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of human Pin1 using Suc-Ala-Glu-Pro-Phe-pNA as substrate by spectrophotometry | Bioorg Med Chem 19: 2797-807 (2011) Article DOI: 10.1016/j.bmc.2011.03.058 BindingDB Entry DOI: 10.7270/Q2SF2WHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||