

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50355815 (CHEMBL1911942) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Curated by ChEMBL | Assay Description Antagonist activity at OX2R expressed in dihydrofolate reductase deficient CHO cells assessed as inhibition of orexin A-induced intracellular calcium... | Bioorg Med Chem Lett 21: 6409-13 (2011) Article DOI: 10.1016/j.bmcl.2011.08.094 BindingDB Entry DOI: 10.7270/Q23F4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50355814 (CHEMBL1911941) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Curated by ChEMBL | Assay Description Antagonist activity at OX2R expressed in dihydrofolate reductase deficient CHO cells assessed as inhibition of orexin A-induced intracellular calcium... | Bioorg Med Chem Lett 21: 6409-13 (2011) Article DOI: 10.1016/j.bmcl.2011.08.094 BindingDB Entry DOI: 10.7270/Q23F4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50355813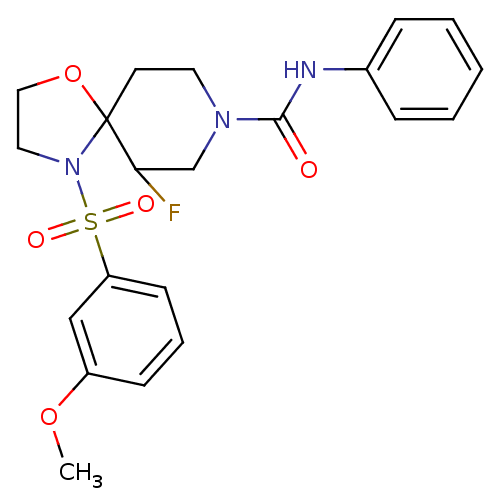 (CHEMBL1911940) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Curated by ChEMBL | Assay Description Antagonist activity at OX2R expressed in dihydrofolate reductase deficient CHO cells assessed as inhibition of orexin A-induced intracellular calcium... | Bioorg Med Chem Lett 21: 6409-13 (2011) Article DOI: 10.1016/j.bmcl.2011.08.094 BindingDB Entry DOI: 10.7270/Q23F4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50419320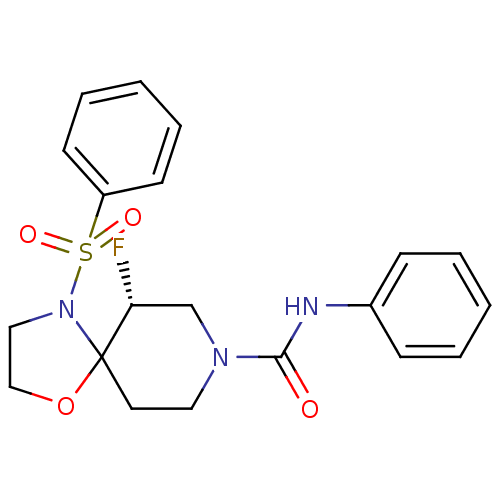 (CHEMBL2092734) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Curated by ChEMBL | Assay Description Antagonist activity at OX2R expressed in dihydrofolate reductase deficient CHO cells assessed as inhibition of orexin A-induced intracellular calcium... | Bioorg Med Chem Lett 21: 6409-13 (2011) Article DOI: 10.1016/j.bmcl.2011.08.094 BindingDB Entry DOI: 10.7270/Q23F4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50419321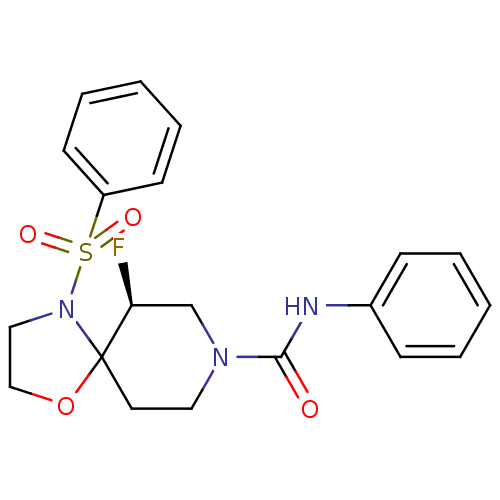 (CHEMBL2092888) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Curated by ChEMBL | Assay Description Antagonist activity at OX2R expressed in dihydrofolate reductase deficient CHO cells assessed as inhibition of orexin A-induced intracellular calcium... | Bioorg Med Chem Lett 21: 6409-13 (2011) Article DOI: 10.1016/j.bmcl.2011.08.094 BindingDB Entry DOI: 10.7270/Q23F4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50355809 (CHEMBL1911936) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Curated by ChEMBL | Assay Description Antagonist activity at OX2R expressed in dihydrofolate reductase deficient CHO cells assessed as inhibition of orexin A-induced intracellular calcium... | Bioorg Med Chem Lett 21: 6409-13 (2011) Article DOI: 10.1016/j.bmcl.2011.08.094 BindingDB Entry DOI: 10.7270/Q23F4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50419320 (CHEMBL2092734) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Curated by ChEMBL | Assay Description Antagonist activity at OX1R expressed in dihydrofolate reductase deficient CHO cells assessed as inhibition of orexin A-induced intracellular calcium... | Bioorg Med Chem Lett 21: 6409-13 (2011) Article DOI: 10.1016/j.bmcl.2011.08.094 BindingDB Entry DOI: 10.7270/Q23F4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50355810 (CHEMBL1911937) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Curated by ChEMBL | Assay Description Antagonist activity at OX2R expressed in dihydrofolate reductase deficient CHO cells assessed as inhibition of orexin A-induced intracellular calcium... | Bioorg Med Chem Lett 21: 6409-13 (2011) Article DOI: 10.1016/j.bmcl.2011.08.094 BindingDB Entry DOI: 10.7270/Q23F4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50355812 (CHEMBL1911939) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Curated by ChEMBL | Assay Description Antagonist activity at OX2R expressed in dihydrofolate reductase deficient CHO cells assessed as inhibition of orexin A-induced intracellular calcium... | Bioorg Med Chem Lett 21: 6409-13 (2011) Article DOI: 10.1016/j.bmcl.2011.08.094 BindingDB Entry DOI: 10.7270/Q23F4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50355815 (CHEMBL1911942) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Curated by ChEMBL | Assay Description Antagonist activity at OX1R expressed in dihydrofolate reductase deficient CHO cells assessed as inhibition of orexin A-induced intracellular calcium... | Bioorg Med Chem Lett 21: 6409-13 (2011) Article DOI: 10.1016/j.bmcl.2011.08.094 BindingDB Entry DOI: 10.7270/Q23F4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50355814 (CHEMBL1911941) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Curated by ChEMBL | Assay Description Antagonist activity at OX1R expressed in dihydrofolate reductase deficient CHO cells assessed as inhibition of orexin A-induced intracellular calcium... | Bioorg Med Chem Lett 21: 6409-13 (2011) Article DOI: 10.1016/j.bmcl.2011.08.094 BindingDB Entry DOI: 10.7270/Q23F4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50355809 (CHEMBL1911936) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Curated by ChEMBL | Assay Description Antagonist activity at OX1R expressed in dihydrofolate reductase deficient CHO cells assessed as inhibition of orexin A-induced intracellular calcium... | Bioorg Med Chem Lett 21: 6409-13 (2011) Article DOI: 10.1016/j.bmcl.2011.08.094 BindingDB Entry DOI: 10.7270/Q23F4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50355813 (CHEMBL1911940) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Curated by ChEMBL | Assay Description Antagonist activity at OX1R expressed in dihydrofolate reductase deficient CHO cells assessed as inhibition of orexin A-induced intracellular calcium... | Bioorg Med Chem Lett 21: 6409-13 (2011) Article DOI: 10.1016/j.bmcl.2011.08.094 BindingDB Entry DOI: 10.7270/Q23F4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50419321 (CHEMBL2092888) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Curated by ChEMBL | Assay Description Antagonist activity at OX1R expressed in dihydrofolate reductase deficient CHO cells assessed as inhibition of orexin A-induced intracellular calcium... | Bioorg Med Chem Lett 21: 6409-13 (2011) Article DOI: 10.1016/j.bmcl.2011.08.094 BindingDB Entry DOI: 10.7270/Q23F4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50355810 (CHEMBL1911937) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Curated by ChEMBL | Assay Description Antagonist activity at OX1R expressed in dihydrofolate reductase deficient CHO cells assessed as inhibition of orexin A-induced intracellular calcium... | Bioorg Med Chem Lett 21: 6409-13 (2011) Article DOI: 10.1016/j.bmcl.2011.08.094 BindingDB Entry DOI: 10.7270/Q23F4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50355812 (CHEMBL1911939) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Curated by ChEMBL | Assay Description Antagonist activity at OX1R expressed in dihydrofolate reductase deficient CHO cells assessed as inhibition of orexin A-induced intracellular calcium... | Bioorg Med Chem Lett 21: 6409-13 (2011) Article DOI: 10.1016/j.bmcl.2011.08.094 BindingDB Entry DOI: 10.7270/Q23F4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||