

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50203126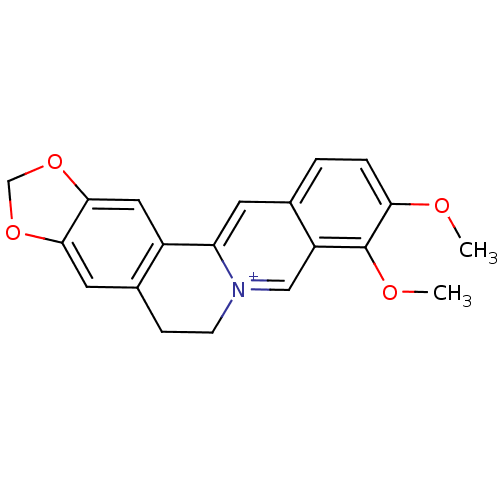 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356913 (CHEMBL1915759) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356928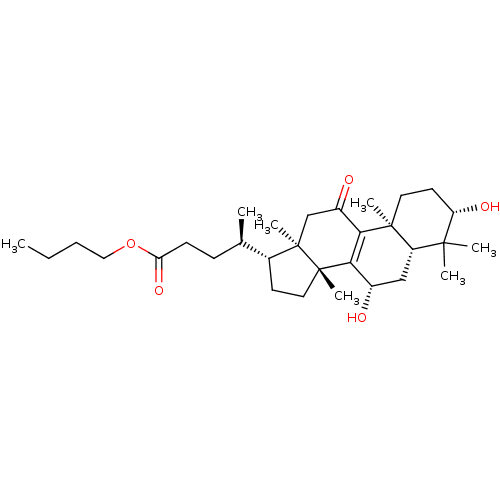 (CHEMBL1915765) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356929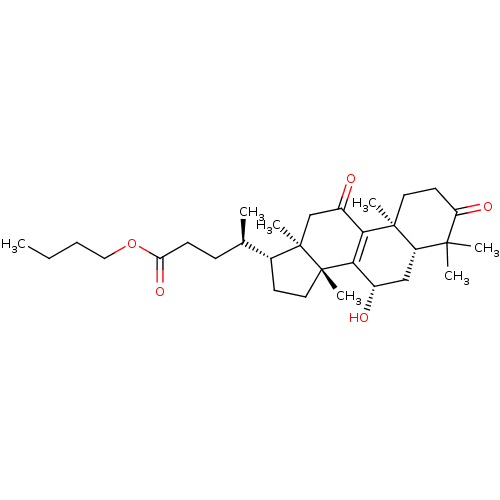 (CHEMBL1915766) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356921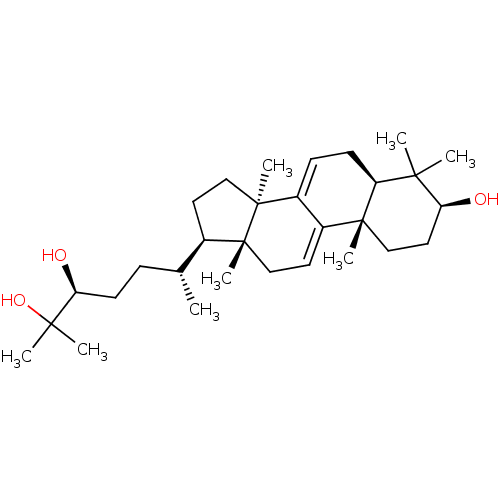 (CHEMBL1915762) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356927 (CHEMBL512830) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356912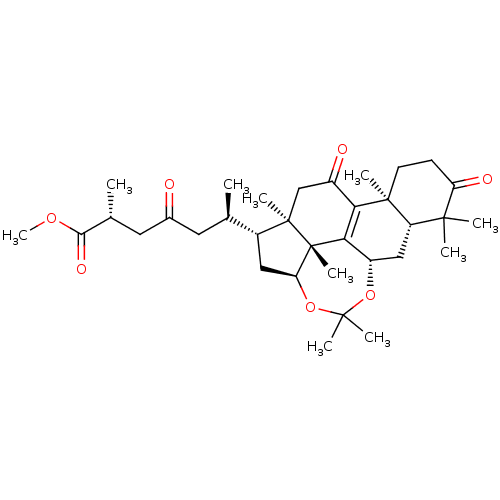 (CHEMBL1915758) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356916 (CHEMBL465514) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50203126 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356919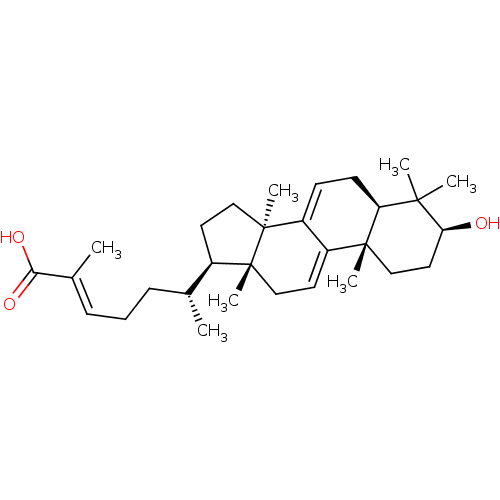 (CHEMBL1915760) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356920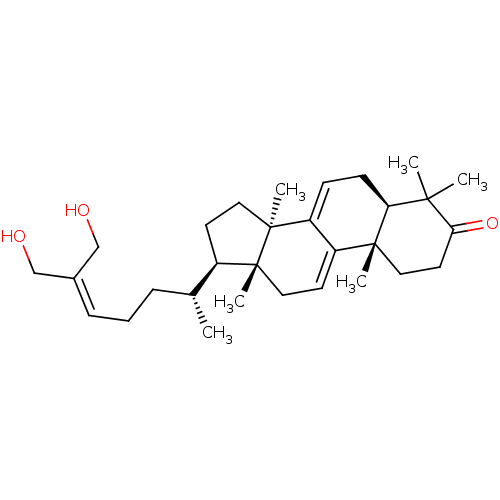 (CHEMBL1915761) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356923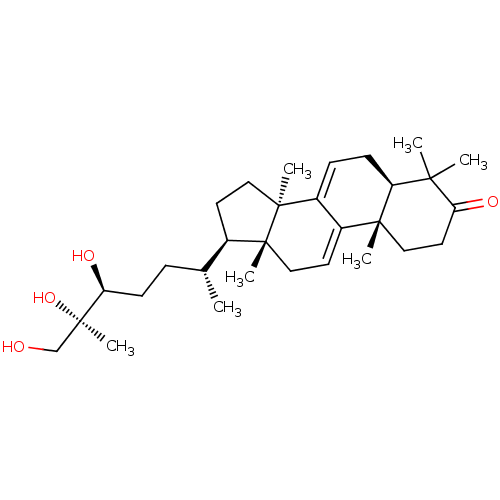 (CHEMBL1915763) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356915 (GANODERIC ACID B) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356917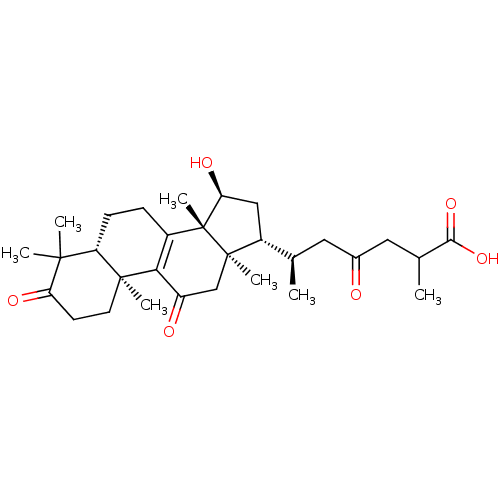 (GANOLUCIDIC ACID A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356926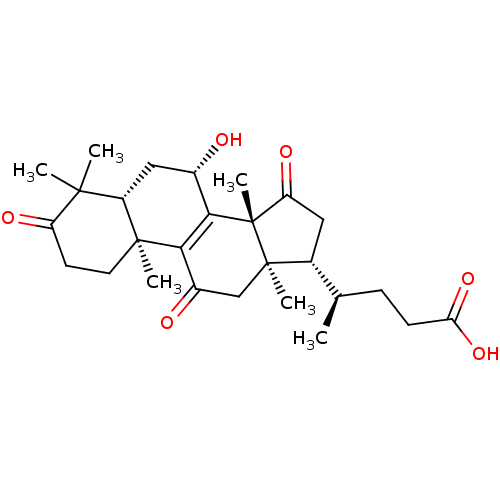 (LUCIDENIC ACID A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356922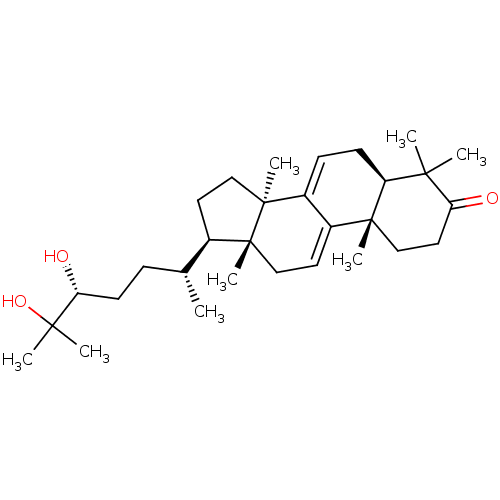 (GANODERMANONDIOL) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356925 (CHEMBL465487) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356918 (GANODEROL B) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356914 (METHYL GANODERATE A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50356924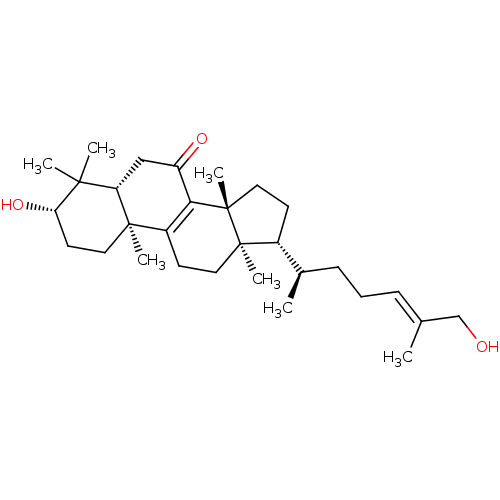 (CHEMBL1915764) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of AChE assessed as hydrolysis of acetylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356924 (CHEMBL1915764) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356925 (CHEMBL465487) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356926 (LUCIDENIC ACID A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356915 (GANODERIC ACID B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356917 (GANOLUCIDIC ACID A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356923 (CHEMBL1915763) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356920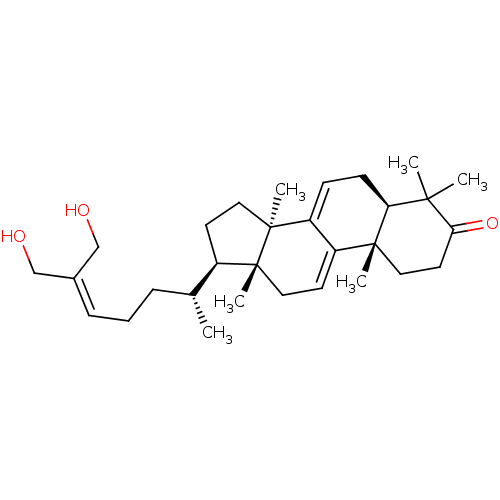 (CHEMBL1915761) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356913 (CHEMBL1915759) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356919 (CHEMBL1915760) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356918 (GANODEROL B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356921 (CHEMBL1915762) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356928 (CHEMBL1915765) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356922 (GANODERMANONDIOL) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356927 (CHEMBL512830) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356916 (CHEMBL465514) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356914 (METHYL GANODERATE A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356929 (CHEMBL1915766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50356912 (CHEMBL1915758) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of BChE assessed as hydrolysis of butrylcholine preincubated for 15 mins measured after 15 mins by colorimetric Ellman assay | Bioorg Med Chem Lett 21: 6603-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.042 BindingDB Entry DOI: 10.7270/Q2M61KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||