

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358117 (CHEMBL1915965) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358116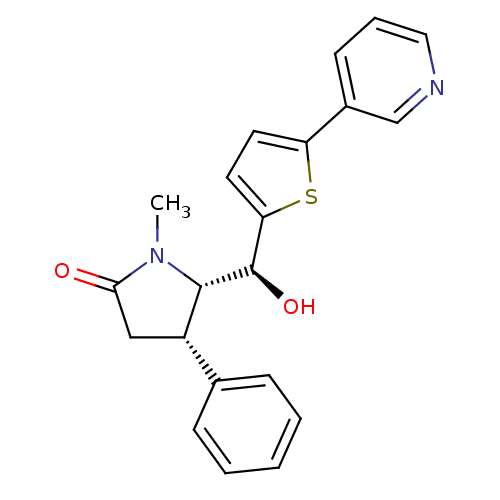 (CHEMBL1915968) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358118 (CHEMBL1915964) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358105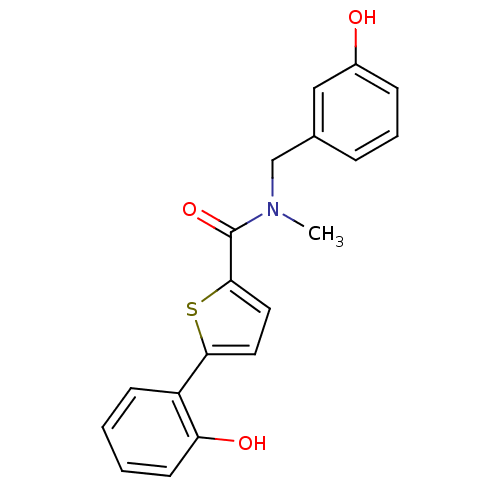 (CHEMBL1915948) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358124 (CHEMBL1915958) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358121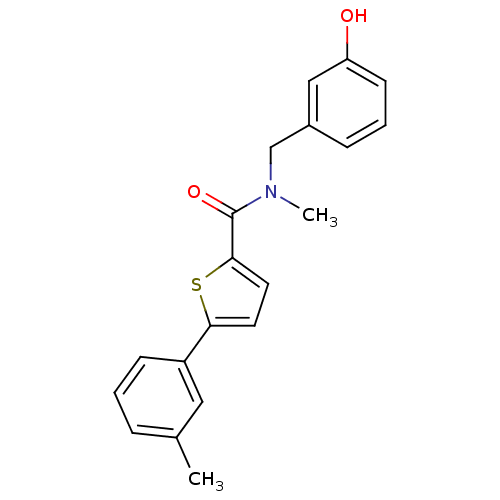 (CHEMBL1915961) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358104 (CHEMBL1915949) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358117 (CHEMBL1915965) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 202 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358129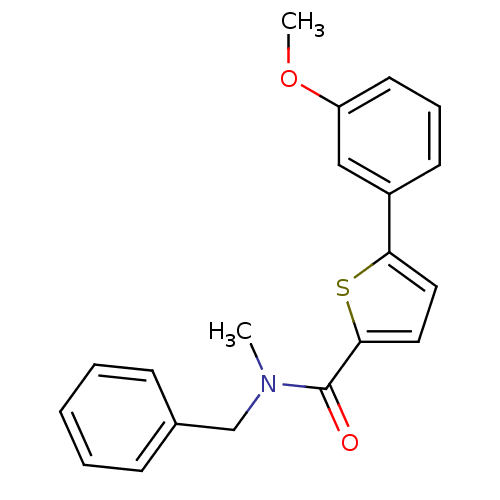 (CHEMBL1915950) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 222 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358122 (CHEMBL1915960) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358112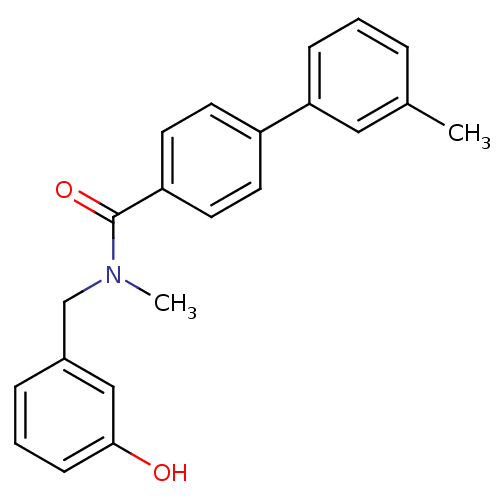 (CHEMBL1915938) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358113 (CHEMBL1915937) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358123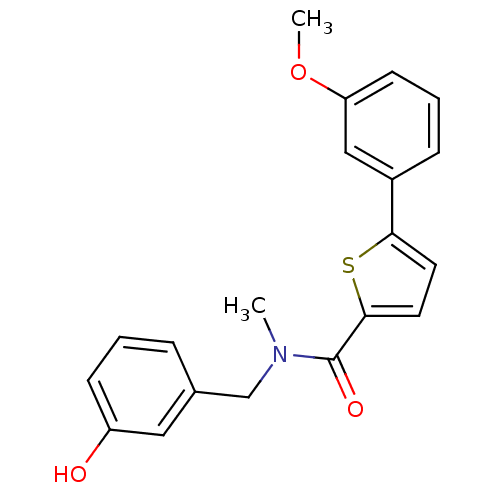 (CHEMBL1915959) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 278 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358120 (CHEMBL1915962) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 326 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358106 (CHEMBL1915946) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 335 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358128 (CHEMBL1915952) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 371 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358109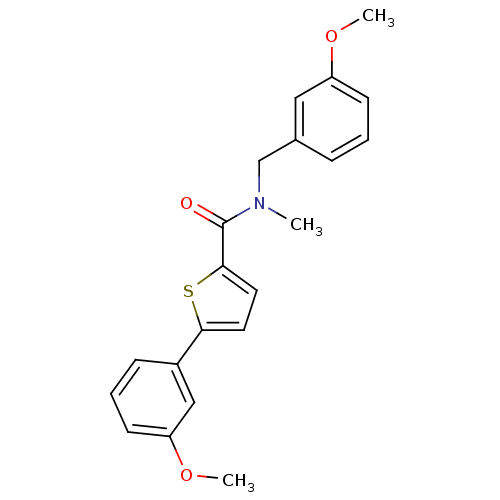 (CHEMBL1915943) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 371 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358108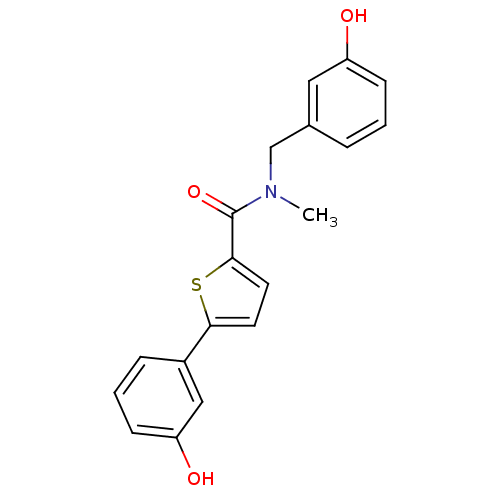 (CHEMBL1915944) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 394 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358119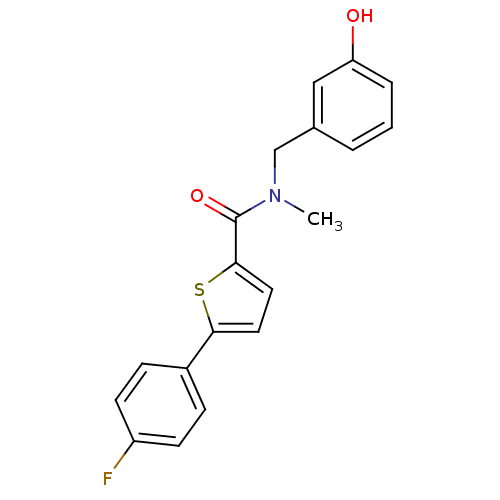 (CHEMBL1915963) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358110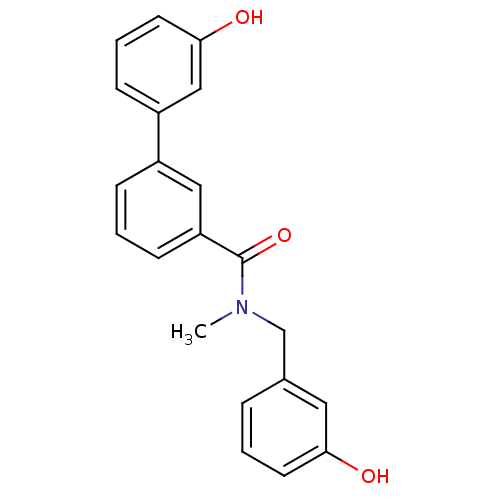 (CHEMBL1915942) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 482 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358115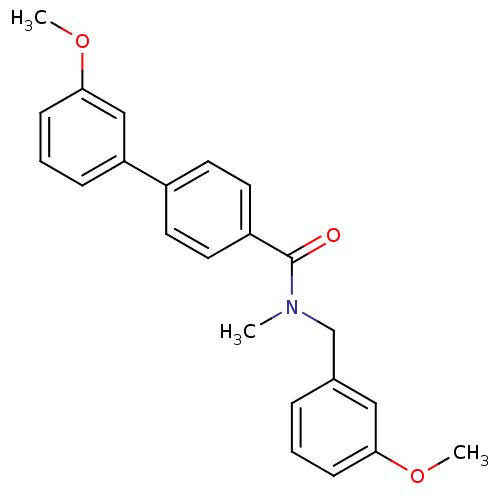 (CHEMBL1915935) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 494 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358107 (CHEMBL1915945) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 496 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358126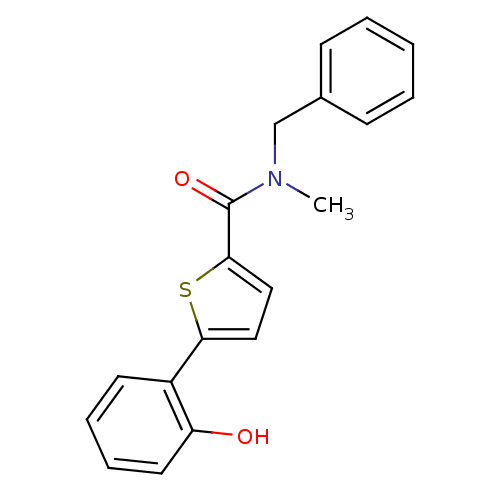 (CHEMBL1915955) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358114 (CHEMBL1915936) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 594 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358111 (CHEMBL1915940) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 639 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358125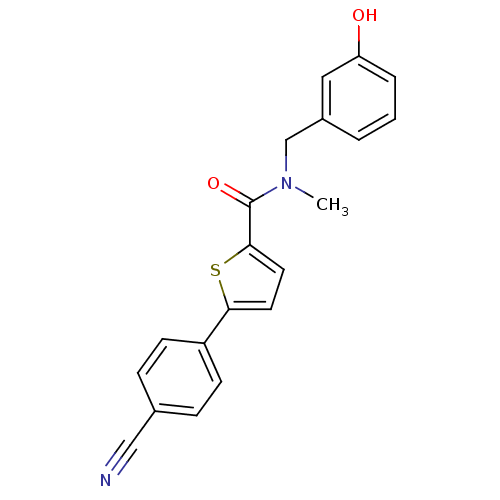 (CHEMBL1915957) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50358127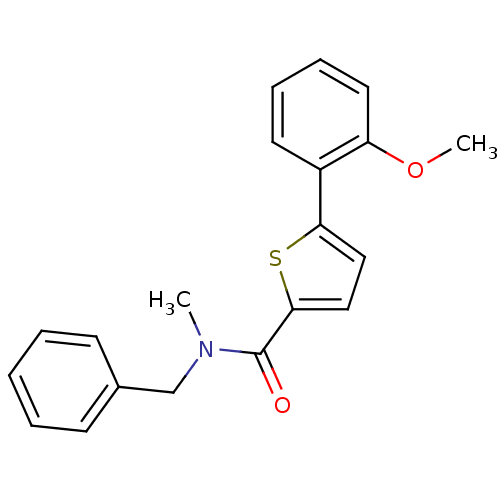 (CHEMBL1915954) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD2 assessed as formation of [2,4,6,7-3H]-estrone using [2,4,6,7-3H]-estradiol as substrate after 20 mins by ra... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358105 (CHEMBL1915948) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358120 (CHEMBL1915962) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358110 (CHEMBL1915942) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358104 (CHEMBL1915949) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358118 (CHEMBL1915964) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358106 (CHEMBL1915946) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358108 (CHEMBL1915944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358111 (CHEMBL1915940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358121 (CHEMBL1915961) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358114 (CHEMBL1915936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358119 (CHEMBL1915963) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358122 (CHEMBL1915960) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358123 (CHEMBL1915959) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358124 (CHEMBL1915958) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358125 (CHEMBL1915957) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358127 (CHEMBL1915954) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358112 (CHEMBL1915938) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358113 (CHEMBL1915937) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358126 (CHEMBL1915955) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358129 (CHEMBL1915950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358109 (CHEMBL1915943) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358115 (CHEMBL1915935) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358128 (CHEMBL1915952) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50358107 (CHEMBL1915945) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human placental 17beta-HSD1 using [2,4,6,7-3H]-estrone as substrate after 10 mins by radio flow detector-based HPLC analysis in presenc... | Eur J Med Chem 46: 5978-90 (2011) Article DOI: 10.1016/j.ejmech.2011.10.010 BindingDB Entry DOI: 10.7270/Q2PN9625 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||