 Found 57 hits of Enzyme Inhibition Constant Data
Found 57 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50209683
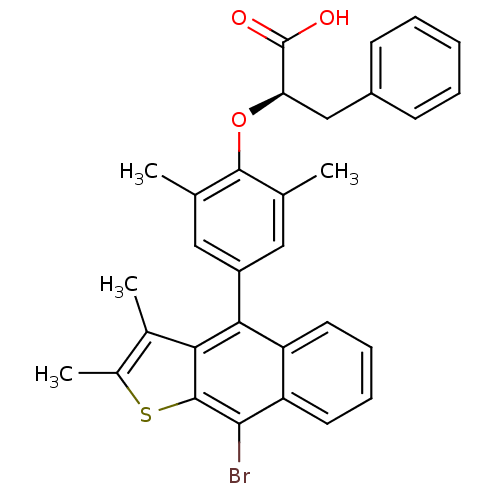
((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...)Show SMILES Cc1sc2c(Br)c3ccccc3c(-c3cc(C)c(O[C@H](Cc4ccccc4)C(O)=O)c(C)c3)c2c1C Show InChI InChI=1S/C31H27BrO3S/c1-17-14-22(15-18(2)29(17)35-25(31(33)34)16-21-10-6-5-7-11-21)27-23-12-8-9-13-24(23)28(32)30-26(27)19(3)20(4)36-30/h5-15,25H,16H2,1-4H3,(H,33,34)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361277
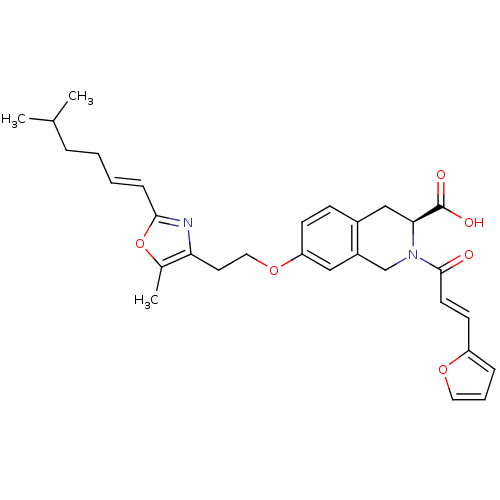
(CHEMBL1933093)Show SMILES CC(C)CC\C=C\c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)\C=C\c2ccco2)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C30H34N2O6/c1-20(2)7-4-5-9-28-31-26(21(3)38-28)14-16-37-25-11-10-22-18-27(30(34)35)32(19-23(22)17-25)29(33)13-12-24-8-6-15-36-24/h5-6,8-13,15,17,20,27H,4,7,14,16,18-19H2,1-3H3,(H,34,35)/b9-5+,13-12+/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361286
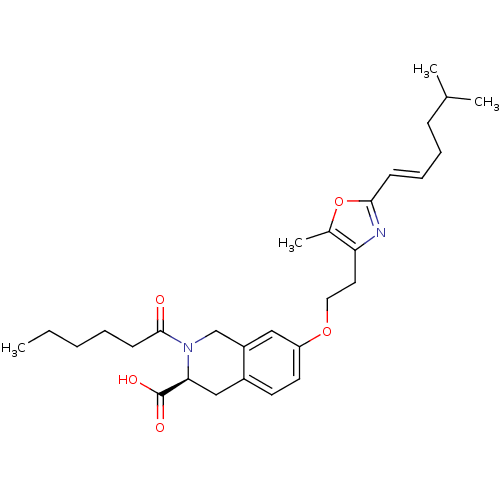
(CHEMBL1935611)Show SMILES CCCCCC(=O)N1Cc2cc(OCCc3nc(\C=C\CCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C29H40N2O5/c1-5-6-7-12-28(32)31-19-23-17-24(14-13-22(23)18-26(31)29(33)34)35-16-15-25-21(4)36-27(30-25)11-9-8-10-20(2)3/h9,11,13-14,17,20,26H,5-8,10,12,15-16,18-19H2,1-4H3,(H,33,34)/b11-9+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361289
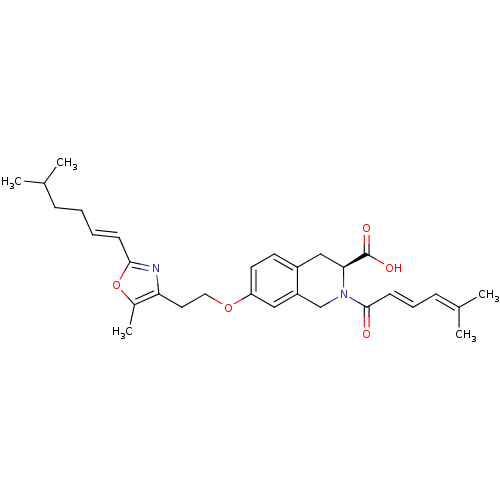
(CHEMBL1935608)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6]\[#6]=[#6]\c1nc(-[#6]-[#6]-[#8]-c2ccc3-[#6]-[#6@H](-[#7](-[#6]-c3c2)-[#6](=O)\[#6]=[#6]\[#6]=[#6](\[#6])-[#6])-[#6](-[#8])=O)c(-[#6])o1 |r| Show InChI InChI=1S/C30H38N2O5/c1-20(2)9-6-7-11-28-31-26(22(5)37-28)15-16-36-25-14-13-23-18-27(30(34)35)32(19-24(23)17-25)29(33)12-8-10-21(3)4/h7-8,10-14,17,20,27H,6,9,15-16,18-19H2,1-5H3,(H,34,35)/b11-7+,12-8+/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361293
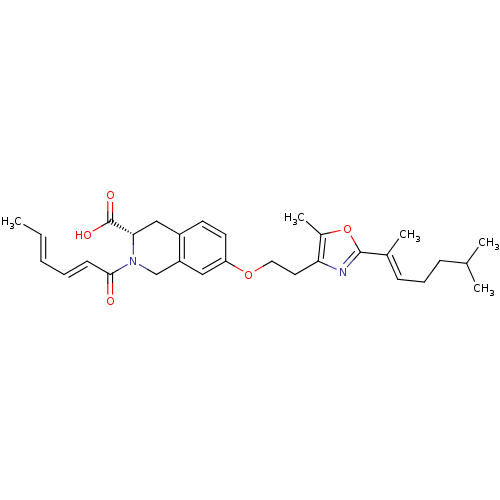
(CHEMBL1935501)Show SMILES C\C=C\C=C\C(=O)N1Cc2cc(OCCc3nc(oc3C)C(\C)=C\CCC(C)C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C30H38N2O5/c1-6-7-8-12-28(33)32-19-24-17-25(14-13-23(24)18-27(32)30(34)35)36-16-15-26-22(5)37-29(31-26)21(4)11-9-10-20(2)3/h6-8,11-14,17,20,27H,9-10,15-16,18-19H2,1-5H3,(H,34,35)/b7-6+,12-8+,21-11+/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361276
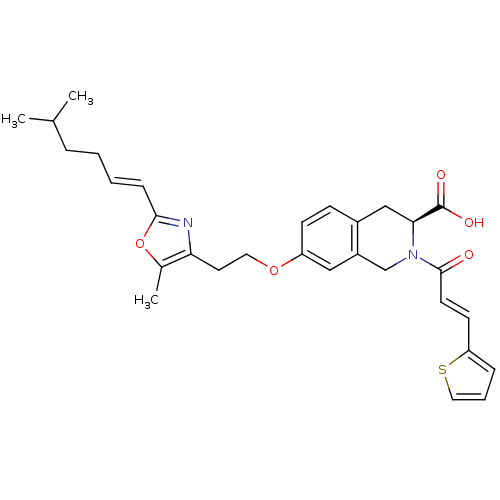
(CHEMBL1935620)Show SMILES CC(C)CC\C=C\c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)\C=C\c2cccs2)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C30H34N2O5S/c1-20(2)7-4-5-9-28-31-26(21(3)37-28)14-15-36-24-11-10-22-18-27(30(34)35)32(19-23(22)17-24)29(33)13-12-25-8-6-16-38-25/h5-6,8-13,16-17,20,27H,4,7,14-15,18-19H2,1-3H3,(H,34,35)/b9-5+,13-12+/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361283
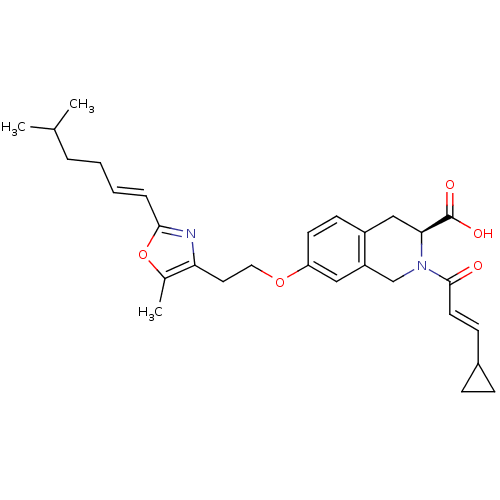
(CHEMBL1935614)Show SMILES CC(C)CC\C=C\c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)\C=C\C2CC2)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C29H36N2O5/c1-19(2)6-4-5-7-27-30-25(20(3)36-27)14-15-35-24-12-11-22-17-26(29(33)34)31(18-23(22)16-24)28(32)13-10-21-8-9-21/h5,7,10-13,16,19,21,26H,4,6,8-9,14-15,17-18H2,1-3H3,(H,33,34)/b7-5+,13-10+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361292
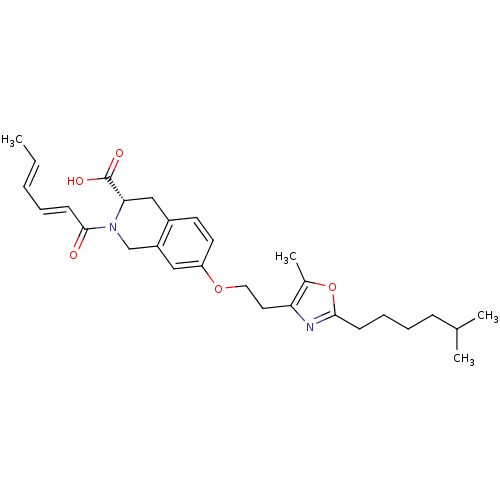
(CHEMBL1935607)Show SMILES C\C=C\C=C\C(=O)N1Cc2cc(OCCc3nc(CCCCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C29H38N2O5/c1-5-6-7-12-28(32)31-19-23-17-24(14-13-22(23)18-26(31)29(33)34)35-16-15-25-21(4)36-27(30-25)11-9-8-10-20(2)3/h5-7,12-14,17,20,26H,8-11,15-16,18-19H2,1-4H3,(H,33,34)/b6-5+,12-7+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361295
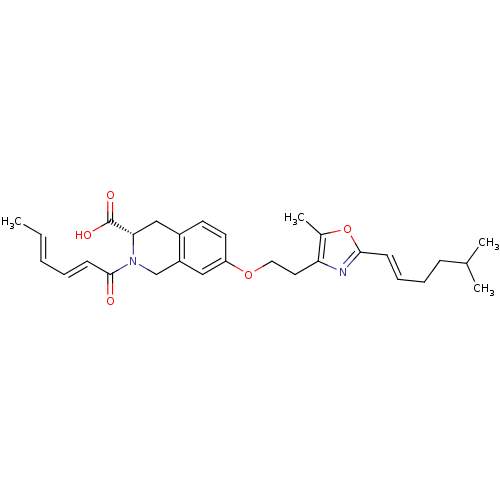
(CHEMBL1935499)Show SMILES C\C=C\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\CCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C29H36N2O5/c1-5-6-7-12-28(32)31-19-23-17-24(14-13-22(23)18-26(31)29(33)34)35-16-15-25-21(4)36-27(30-25)11-9-8-10-20(2)3/h5-7,9,11-14,17,20,26H,8,10,15-16,18-19H2,1-4H3,(H,33,34)/b6-5+,11-9+,12-7+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361287
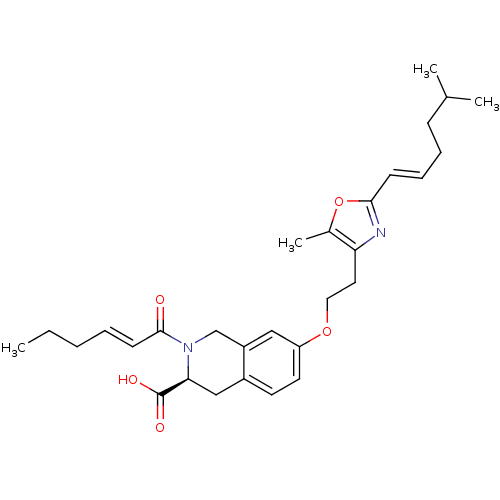
(CHEMBL1935610)Show SMILES CCC\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\CCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C29H38N2O5/c1-5-6-7-12-28(32)31-19-23-17-24(14-13-22(23)18-26(31)29(33)34)35-16-15-25-21(4)36-27(30-25)11-9-8-10-20(2)3/h7,9,11-14,17,20,26H,5-6,8,10,15-16,18-19H2,1-4H3,(H,33,34)/b11-9+,12-7+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361298
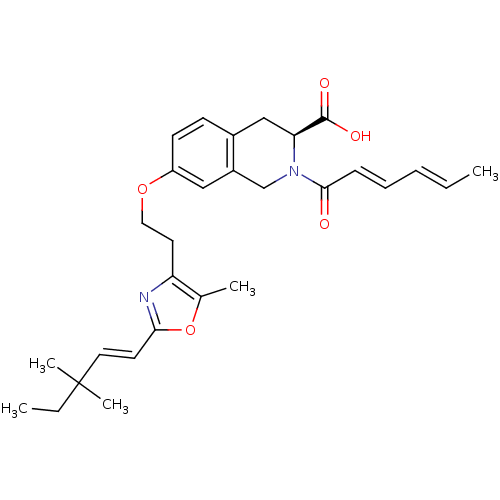
(CHEMBL1935496)Show SMILES CCC(C)(C)\C=C\c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)\C=C\C=C\C)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C29H36N2O5/c1-6-8-9-10-27(32)31-19-22-17-23(12-11-21(22)18-25(31)28(33)34)35-16-14-24-20(3)36-26(30-24)13-15-29(4,5)7-2/h6,8-13,15,17,25H,7,14,16,18-19H2,1-5H3,(H,33,34)/b8-6+,10-9+,15-13+/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361296
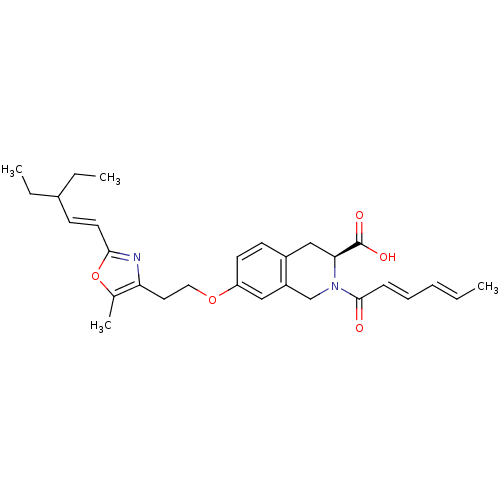
(CHEMBL1935498)Show SMILES CCC(CC)\C=C\c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)\C=C\C=C\C)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C29H36N2O5/c1-5-8-9-10-28(32)31-19-23-17-24(13-12-22(23)18-26(31)29(33)34)35-16-15-25-20(4)36-27(30-25)14-11-21(6-2)7-3/h5,8-14,17,21,26H,6-7,15-16,18-19H2,1-4H3,(H,33,34)/b8-5+,10-9+,14-11+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361294
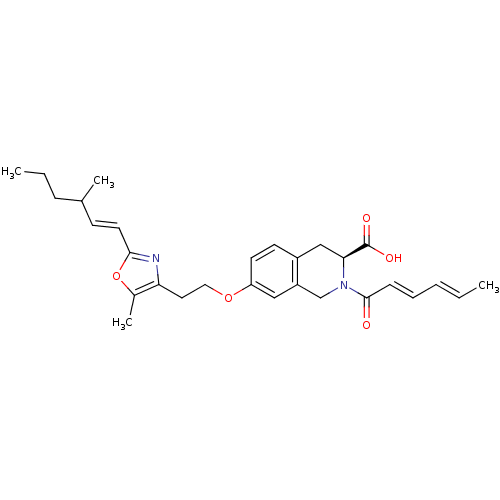
(CHEMBL1935500)Show SMILES CCCC(C)\C=C\c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)\C=C\C=C\C)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C29H36N2O5/c1-5-7-8-10-28(32)31-19-23-17-24(13-12-22(23)18-26(31)29(33)34)35-16-15-25-21(4)36-27(30-25)14-11-20(3)9-6-2/h5,7-8,10-14,17,20,26H,6,9,15-16,18-19H2,1-4H3,(H,33,34)/b7-5+,10-8+,14-11+/t20?,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361285
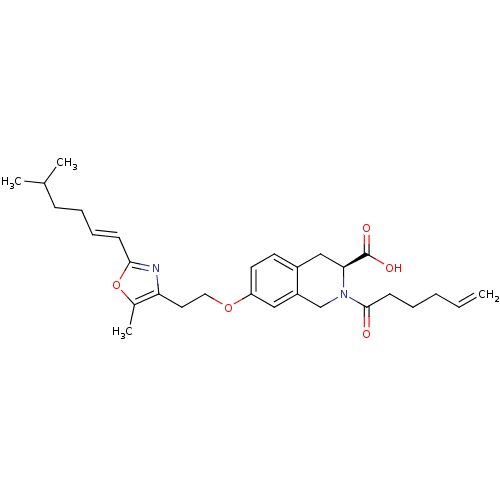
(CHEMBL1935612)Show SMILES CC(C)CC\C=C\c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)CCCC=C)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C29H38N2O5/c1-5-6-7-12-28(32)31-19-23-17-24(14-13-22(23)18-26(31)29(33)34)35-16-15-25-21(4)36-27(30-25)11-9-8-10-20(2)3/h5,9,11,13-14,17,20,26H,1,6-8,10,12,15-16,18-19H2,2-4H3,(H,33,34)/b11-9+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361284

(CHEMBL1935613)Show SMILES CC(C)CC\C=C\c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)CCC=C)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C28H36N2O5/c1-5-6-11-27(31)30-18-22-16-23(13-12-21(22)17-25(30)28(32)33)34-15-14-24-20(4)35-26(29-24)10-8-7-9-19(2)3/h5,8,10,12-13,16,19,25H,1,6-7,9,11,14-15,17-18H2,2-4H3,(H,32,33)/b10-8+/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361297
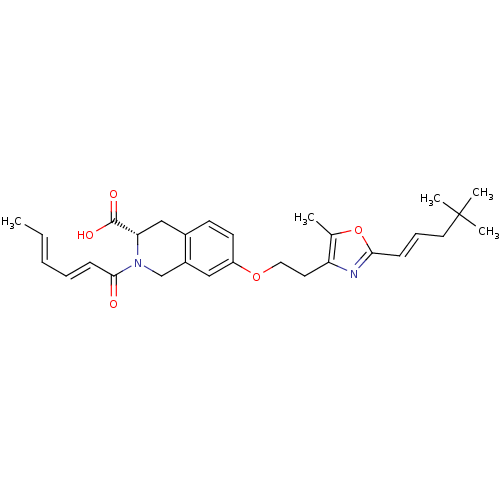
(CHEMBL1935497)Show SMILES C\C=C\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\CC(C)(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C29H36N2O5/c1-6-7-8-11-27(32)31-19-22-17-23(13-12-21(22)18-25(31)28(33)34)35-16-14-24-20(2)36-26(30-24)10-9-15-29(3,4)5/h6-13,17,25H,14-16,18-19H2,1-5H3,(H,33,34)/b7-6+,10-9+,11-8+/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361290
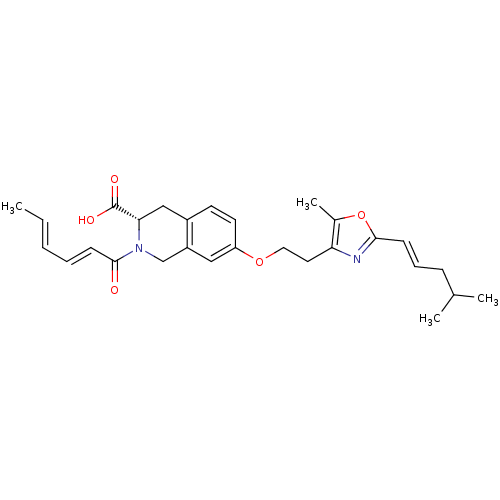
(CHEMBL1935495)Show SMILES C\C=C\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\CC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C28H34N2O5/c1-5-6-7-11-27(31)30-18-22-16-23(13-12-21(22)17-25(30)28(32)33)34-15-14-24-20(4)35-26(29-24)10-8-9-19(2)3/h5-8,10-13,16,19,25H,9,14-15,17-18H2,1-4H3,(H,32,33)/b6-5+,10-8+,11-7+/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361288
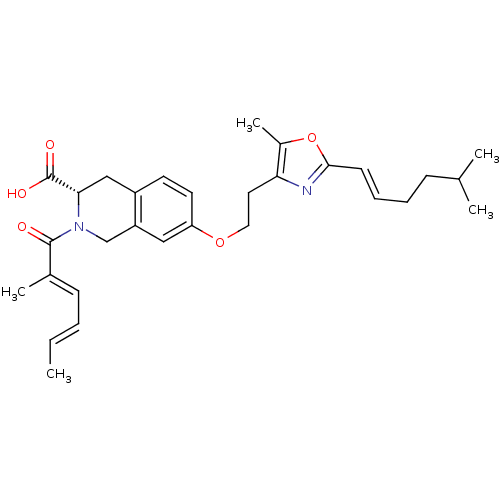
(CHEMBL1935609)Show SMILES C\C=C\C=C(/C)C(=O)N1Cc2cc(OCCc3nc(\C=C\CCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C30H38N2O5/c1-6-7-11-21(4)29(33)32-19-24-17-25(14-13-23(24)18-27(32)30(34)35)36-16-15-26-22(5)37-28(31-26)12-9-8-10-20(2)3/h6-7,9,11-14,17,20,27H,8,10,15-16,18-19H2,1-5H3,(H,34,35)/b7-6+,12-9+,21-11+/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361291
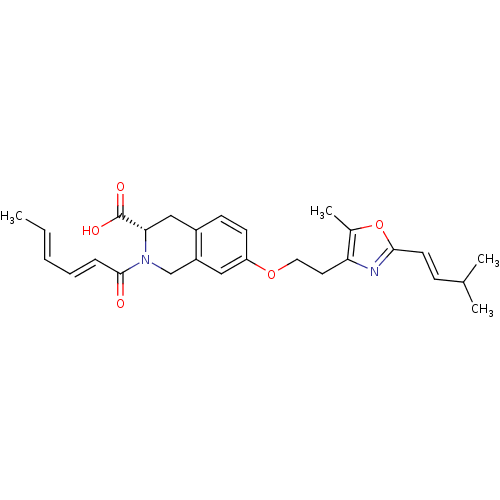
(CHEMBL1935493)Show SMILES C\C=C\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\C(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C27H32N2O5/c1-5-6-7-8-26(30)29-17-21-15-22(11-10-20(21)16-24(29)27(31)32)33-14-13-23-19(4)34-25(28-23)12-9-18(2)3/h5-12,15,18,24H,13-14,16-17H2,1-4H3,(H,31,32)/b6-5+,8-7+,12-9+/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361299

(CHEMBL1935494)Show SMILES C\C=C\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\C(C)(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C28H34N2O5/c1-6-7-8-9-26(31)30-18-21-16-22(11-10-20(21)17-24(30)27(32)33)34-15-13-23-19(2)35-25(29-23)12-14-28(3,4)5/h6-12,14,16,24H,13,15,17-18H2,1-5H3,(H,32,33)/b7-6+,9-8+,14-12+/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361279
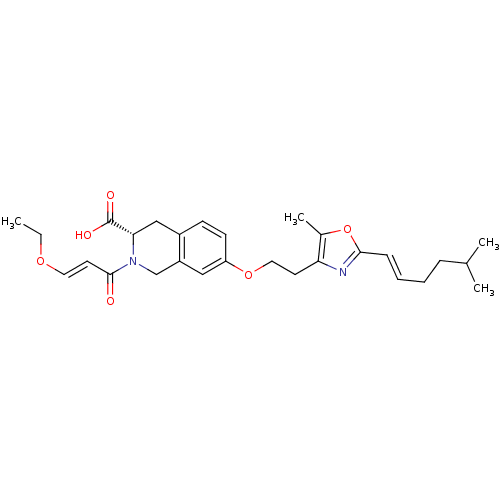
(CHEMBL1935618)Show SMILES CCO\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\CCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C28H36N2O6/c1-5-34-14-13-27(31)30-18-22-16-23(11-10-21(22)17-25(30)28(32)33)35-15-12-24-20(4)36-26(29-24)9-7-6-8-19(2)3/h7,9-11,13-14,16,19,25H,5-6,8,12,15,17-18H2,1-4H3,(H,32,33)/b9-7+,14-13+/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361282
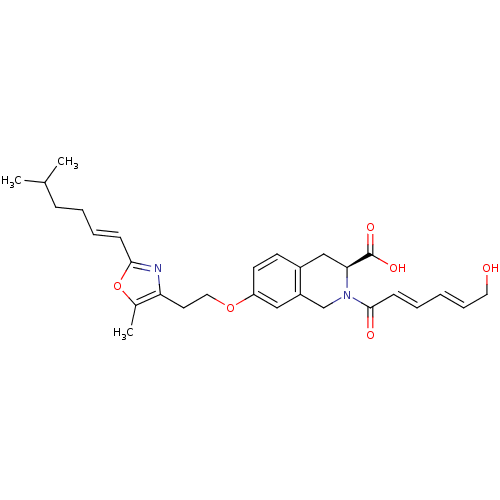
(CHEMBL1935615)Show SMILES CC(C)CC\C=C\c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)\C=C\C=C\CO)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C29H36N2O6/c1-20(2)9-6-7-10-27-30-25(21(3)37-27)14-16-36-24-13-12-22-18-26(29(34)35)31(19-23(22)17-24)28(33)11-5-4-8-15-32/h4-5,7-8,10-13,17,20,26,32H,6,9,14-16,18-19H2,1-3H3,(H,34,35)/b8-4+,10-7+,11-5+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361278
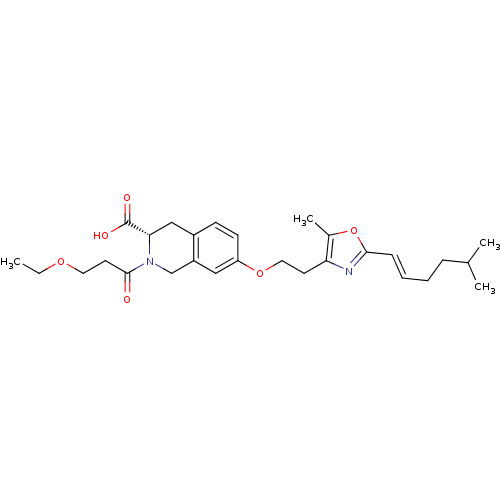
(CHEMBL1935619)Show SMILES CCOCCC(=O)N1Cc2cc(OCCc3nc(\C=C\CCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C28H38N2O6/c1-5-34-14-13-27(31)30-18-22-16-23(11-10-21(22)17-25(30)28(32)33)35-15-12-24-20(4)36-26(29-24)9-7-6-8-19(2)3/h7,9-11,16,19,25H,5-6,8,12-15,17-18H2,1-4H3,(H,32,33)/b9-7+/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361280
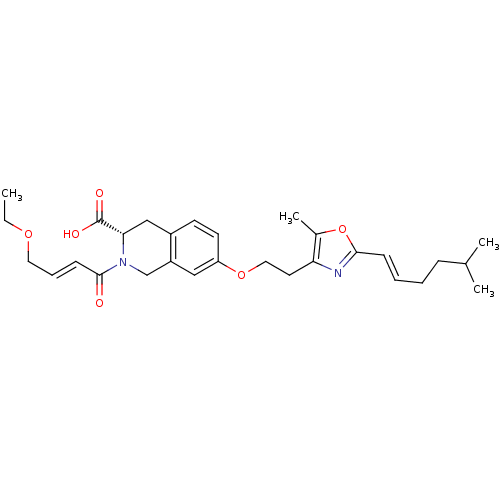
(CHEMBL1935617)Show SMILES CCOC\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\CCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C29H38N2O6/c1-5-35-15-8-11-28(32)31-19-23-17-24(13-12-22(23)18-26(31)29(33)34)36-16-14-25-21(4)37-27(30-25)10-7-6-9-20(2)3/h7-8,10-13,17,20,26H,5-6,9,14-16,18-19H2,1-4H3,(H,33,34)/b10-7+,11-8+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361300

(CHEMBL1935492)Show SMILES CC\C=C(/C)c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)\C=C\C=C\C)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C27H32N2O5/c1-5-7-8-10-25(30)29-17-21-15-22(12-11-20(21)16-24(29)27(31)32)33-14-13-23-19(4)34-26(28-23)18(3)9-6-2/h5,7-12,15,24H,6,13-14,16-17H2,1-4H3,(H,31,32)/b7-5+,10-8+,18-9+/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361301
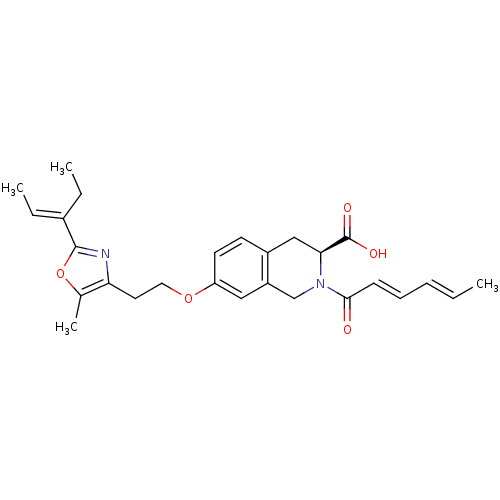
(CHEMBL1935491)Show SMILES CC\C(=C/C)c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)\C=C\C=C\C)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C27H32N2O5/c1-5-8-9-10-25(30)29-17-21-15-22(12-11-20(21)16-24(29)27(31)32)33-14-13-23-18(4)34-26(28-23)19(6-2)7-3/h5-6,8-12,15,24H,7,13-14,16-17H2,1-4H3,(H,31,32)/b8-5+,10-9+,19-6+/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50361281
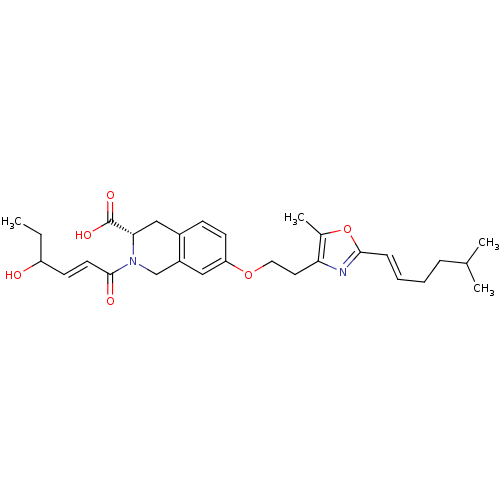
(CHEMBL1935616)Show SMILES CCC(O)\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\CCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C29H38N2O6/c1-5-23(32)11-13-28(33)31-18-22-16-24(12-10-21(22)17-26(31)29(34)35)36-15-14-25-20(4)37-27(30-25)9-7-6-8-19(2)3/h7,9-13,16,19,23,26,32H,5-6,8,14-15,17-18H2,1-4H3,(H,34,35)/b9-7+,13-11+/t23?,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361300

(CHEMBL1935492)Show SMILES CC\C=C(/C)c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)\C=C\C=C\C)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C27H32N2O5/c1-5-7-8-10-25(30)29-17-21-15-22(12-11-20(21)16-24(29)27(31)32)33-14-13-23-19(4)34-26(28-23)18(3)9-6-2/h5,7-12,15,24H,6,13-14,16-17H2,1-4H3,(H,31,32)/b7-5+,10-8+,18-9+/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361299

(CHEMBL1935494)Show SMILES C\C=C\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\C(C)(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C28H34N2O5/c1-6-7-8-9-26(31)30-18-21-16-22(11-10-20(21)17-24(30)27(32)33)34-15-13-23-19(2)35-25(29-23)12-14-28(3,4)5/h6-12,14,16,24H,13,15,17-18H2,1-5H3,(H,32,33)/b7-6+,9-8+,14-12+/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 540 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361295

(CHEMBL1935499)Show SMILES C\C=C\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\CCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C29H36N2O5/c1-5-6-7-12-28(32)31-19-23-17-24(14-13-22(23)18-26(31)29(33)34)35-16-15-25-21(4)36-27(30-25)11-9-8-10-20(2)3/h5-7,9,11-14,17,20,26H,8,10,15-16,18-19H2,1-4H3,(H,33,34)/b6-5+,11-9+,12-7+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361278

(CHEMBL1935619)Show SMILES CCOCCC(=O)N1Cc2cc(OCCc3nc(\C=C\CCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C28H38N2O6/c1-5-34-14-13-27(31)30-18-22-16-23(11-10-21(22)17-25(30)28(32)33)35-15-12-24-20(4)36-26(29-24)9-7-6-8-19(2)3/h7,9-11,16,19,25H,5-6,8,12-15,17-18H2,1-4H3,(H,32,33)/b9-7+/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361281

(CHEMBL1935616)Show SMILES CCC(O)\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\CCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C29H38N2O6/c1-5-23(32)11-13-28(33)31-18-22-16-24(12-10-21(22)17-26(31)29(34)35)36-15-14-25-20(4)37-27(30-25)9-7-6-8-19(2)3/h7,9-13,16,19,23,26,32H,5-6,8,14-15,17-18H2,1-4H3,(H,34,35)/b9-7+,13-11+/t23?,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361289

(CHEMBL1935608)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6]\[#6]=[#6]\c1nc(-[#6]-[#6]-[#8]-c2ccc3-[#6]-[#6@H](-[#7](-[#6]-c3c2)-[#6](=O)\[#6]=[#6]\[#6]=[#6](\[#6])-[#6])-[#6](-[#8])=O)c(-[#6])o1 |r| Show InChI InChI=1S/C30H38N2O5/c1-20(2)9-6-7-11-28-31-26(22(5)37-28)15-16-36-25-14-13-23-18-27(30(34)35)32(19-24(23)17-25)29(33)12-8-10-21(3)4/h7-8,10-14,17,20,27H,6,9,15-16,18-19H2,1-5H3,(H,34,35)/b11-7+,12-8+/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361297

(CHEMBL1935497)Show SMILES C\C=C\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\CC(C)(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C29H36N2O5/c1-6-7-8-11-27(32)31-19-22-17-23(13-12-21(22)18-25(31)28(33)34)35-16-14-24-20(2)36-26(30-24)10-9-15-29(3,4)5/h6-13,17,25H,14-16,18-19H2,1-5H3,(H,33,34)/b7-6+,10-9+,11-8+/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361301

(CHEMBL1935491)Show SMILES CC\C(=C/C)c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)\C=C\C=C\C)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C27H32N2O5/c1-5-8-9-10-25(30)29-17-21-15-22(12-11-20(21)16-24(29)27(31)32)33-14-13-23-18(4)34-26(28-23)19(6-2)7-3/h5-6,8-12,15,24H,7,13-14,16-17H2,1-4H3,(H,31,32)/b8-5+,10-9+,19-6+/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361276

(CHEMBL1935620)Show SMILES CC(C)CC\C=C\c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)\C=C\c2cccs2)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C30H34N2O5S/c1-20(2)7-4-5-9-28-31-26(21(3)37-28)14-15-36-24-11-10-22-18-27(30(34)35)32(19-23(22)17-24)29(33)13-12-25-8-6-16-38-25/h5-6,8-13,16-17,20,27H,4,7,14-15,18-19H2,1-3H3,(H,34,35)/b9-5+,13-12+/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361277

(CHEMBL1933093)Show SMILES CC(C)CC\C=C\c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)\C=C\c2ccco2)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C30H34N2O6/c1-20(2)7-4-5-9-28-31-26(21(3)38-28)14-16-37-25-11-10-22-18-27(30(34)35)32(19-23(22)17-25)29(33)13-12-24-8-6-15-36-24/h5-6,8-13,15,17,20,27H,4,7,14,16,18-19H2,1-3H3,(H,34,35)/b9-5+,13-12+/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361285

(CHEMBL1935612)Show SMILES CC(C)CC\C=C\c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)CCCC=C)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C29H38N2O5/c1-5-6-7-12-28(32)31-19-23-17-24(14-13-22(23)18-26(31)29(33)34)35-16-15-25-21(4)36-27(30-25)11-9-8-10-20(2)3/h5,9,11,13-14,17,20,26H,1,6-8,10,12,15-16,18-19H2,2-4H3,(H,33,34)/b11-9+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361294

(CHEMBL1935500)Show SMILES CCCC(C)\C=C\c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)\C=C\C=C\C)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C29H36N2O5/c1-5-7-8-10-28(32)31-19-23-17-24(13-12-22(23)18-26(31)29(33)34)35-16-15-25-21(4)36-27(30-25)14-11-20(3)9-6-2/h5,7-8,10-14,17,20,26H,6,9,15-16,18-19H2,1-4H3,(H,33,34)/b7-5+,10-8+,14-11+/t20?,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361296

(CHEMBL1935498)Show SMILES CCC(CC)\C=C\c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)\C=C\C=C\C)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C29H36N2O5/c1-5-8-9-10-28(32)31-19-23-17-24(13-12-22(23)18-26(31)29(33)34)35-16-15-25-20(4)36-27(30-25)14-11-21(6-2)7-3/h5,8-14,17,21,26H,6-7,15-16,18-19H2,1-4H3,(H,33,34)/b8-5+,10-9+,14-11+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 760 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361298

(CHEMBL1935496)Show SMILES CCC(C)(C)\C=C\c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)\C=C\C=C\C)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C29H36N2O5/c1-6-8-9-10-27(32)31-19-22-17-23(12-11-21(22)18-25(31)28(33)34)35-16-14-24-20(3)36-26(30-24)13-15-29(4,5)7-2/h6,8-13,15,17,25H,7,14,16,18-19H2,1-5H3,(H,33,34)/b8-6+,10-9+,15-13+/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 760 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361280

(CHEMBL1935617)Show SMILES CCOC\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\CCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C29H38N2O6/c1-5-35-15-8-11-28(32)31-19-23-17-24(13-12-22(23)18-26(31)29(33)34)36-16-14-25-21(4)37-27(30-25)10-7-6-9-20(2)3/h7-8,10-13,17,20,26H,5-6,9,14-16,18-19H2,1-4H3,(H,33,34)/b10-7+,11-8+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361288

(CHEMBL1935609)Show SMILES C\C=C\C=C(/C)C(=O)N1Cc2cc(OCCc3nc(\C=C\CCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C30H38N2O5/c1-6-7-11-21(4)29(33)32-19-24-17-25(14-13-23(24)18-27(32)30(34)35)36-16-15-26-22(5)37-28(31-26)12-9-8-10-20(2)3/h6-7,9,11-14,17,20,27H,8,10,15-16,18-19H2,1-5H3,(H,34,35)/b7-6+,12-9+,21-11+/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361286

(CHEMBL1935611)Show SMILES CCCCCC(=O)N1Cc2cc(OCCc3nc(\C=C\CCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C29H40N2O5/c1-5-6-7-12-28(32)31-19-23-17-24(14-13-22(23)18-26(31)29(33)34)35-16-15-25-21(4)36-27(30-25)11-9-8-10-20(2)3/h9,11,13-14,17,20,26H,5-8,10,12,15-16,18-19H2,1-4H3,(H,33,34)/b11-9+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361284

(CHEMBL1935613)Show SMILES CC(C)CC\C=C\c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)CCC=C)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C28H36N2O5/c1-5-6-11-27(31)30-18-22-16-23(13-12-21(22)17-25(30)28(32)33)34-15-14-24-20(4)35-26(29-24)10-8-7-9-19(2)3/h5,8,10,12-13,16,19,25H,1,6-7,9,11,14-15,17-18H2,2-4H3,(H,32,33)/b10-8+/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361283

(CHEMBL1935614)Show SMILES CC(C)CC\C=C\c1nc(CCOc2ccc3C[C@H](N(Cc3c2)C(=O)\C=C\C2CC2)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C29H36N2O5/c1-19(2)6-4-5-7-27-30-25(20(3)36-27)14-15-35-24-12-11-22-17-26(29(33)34)31(18-23(22)16-24)28(32)13-10-21-8-9-21/h5,7,10-13,16,19,21,26H,4,6,8-9,14-15,17-18H2,1-3H3,(H,33,34)/b7-5+,13-10+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50361291

(CHEMBL1935493)Show SMILES C\C=C\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\C(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C27H32N2O5/c1-5-6-7-8-26(30)29-17-21-15-22(11-10-20(21)16-24(29)27(31)32)33-14-13-23-19(4)34-25(28-23)12-9-18(2)3/h5-12,15,18,24H,13-14,16-17H2,1-4H3,(H,31,32)/b6-5+,8-7+,12-9+/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARalpha expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361292

(CHEMBL1935607)Show SMILES C\C=C\C=C\C(=O)N1Cc2cc(OCCc3nc(CCCCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C29H38N2O5/c1-5-6-7-12-28(32)31-19-23-17-24(14-13-22(23)18-26(31)29(33)34)35-16-15-25-21(4)36-27(30-25)11-9-8-10-20(2)3/h5-7,12-14,17,20,26H,8-11,15-16,18-19H2,1-4H3,(H,33,34)/b6-5+,12-7+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50361290

(CHEMBL1935495)Show SMILES C\C=C\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\CC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C28H34N2O5/c1-5-6-7-11-27(31)30-18-22-16-23(13-12-21(22)17-25(30)28(32)33)34-15-14-24-20(4)35-26(29-24)10-8-9-19(2)3/h5-8,10-13,16,19,25H,9,14-15,17-18H2,1-4H3,(H,32,33)/b6-5+,10-8+,11-7+/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARalpha expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50361289

(CHEMBL1935608)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6]\[#6]=[#6]\c1nc(-[#6]-[#6]-[#8]-c2ccc3-[#6]-[#6@H](-[#7](-[#6]-c3c2)-[#6](=O)\[#6]=[#6]\[#6]=[#6](\[#6])-[#6])-[#6](-[#8])=O)c(-[#6])o1 |r| Show InChI InChI=1S/C30H38N2O5/c1-20(2)9-6-7-11-28-31-26(22(5)37-28)15-16-36-25-14-13-23-18-27(30(34)35)32(19-24(23)17-25)29(33)12-8-10-21(3)4/h7-8,10-14,17,20,27H,6,9,15-16,18-19H2,1-5H3,(H,34,35)/b11-7+,12-8+/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARalpha expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361291

(CHEMBL1935493)Show SMILES C\C=C\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\C(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C27H32N2O5/c1-5-6-7-8-26(30)29-17-21-15-22(11-10-20(21)16-24(29)27(31)32)33-14-13-23-19(4)34-25(28-23)12-9-18(2)3/h5-12,15,18,24H,13-14,16-17H2,1-4H3,(H,31,32)/b6-5+,8-7+,12-9+/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 670 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361290

(CHEMBL1935495)Show SMILES C\C=C\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\CC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C28H34N2O5/c1-5-6-7-11-27(31)30-18-22-16-23(13-12-21(22)17-25(30)28(32)33)34-15-14-24-20(4)35-26(29-24)10-8-9-19(2)3/h5-8,10-13,16,19,25H,9,14-15,17-18H2,1-4H3,(H,32,33)/b6-5+,10-8+,11-7+/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361279

(CHEMBL1935618)Show SMILES CCO\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\CCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C28H36N2O6/c1-5-34-14-13-27(31)30-18-22-16-23(11-10-21(22)17-25(30)28(32)33)35-15-12-24-20(4)36-26(29-24)9-7-6-8-19(2)3/h7,9-11,13-14,16,19,25H,5-6,8,12,15,17-18H2,1-4H3,(H,32,33)/b9-7+,14-13+/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361287

(CHEMBL1935610)Show SMILES CCC\C=C\C(=O)N1Cc2cc(OCCc3nc(\C=C\CCC(C)C)oc3C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C29H38N2O5/c1-5-6-7-12-28(32)31-19-23-17-24(14-13-22(23)18-26(31)29(33)34)35-16-15-25-21(4)36-27(30-25)11-9-8-10-20(2)3/h7,9,11-14,17,20,26H,5-6,8,10,15-16,18-19H2,1-4H3,(H,33,34)/b11-9+,12-7+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361293

(CHEMBL1935501)Show SMILES C\C=C\C=C\C(=O)N1Cc2cc(OCCc3nc(oc3C)C(\C)=C\CCC(C)C)ccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C30H38N2O5/c1-6-7-8-12-28(33)32-19-24-17-25(14-13-23(24)18-27(32)30(34)35)36-16-15-26-22(5)37-29(31-26)21(4)11-9-10-20(2)3/h6-8,11-14,17,20,27H,9-10,15-16,18-19H2,1-5H3,(H,34,35)/b7-6+,12-8+,21-11+/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Transactivation of human full length PPARgamma expressed in COS1 cells co-transfected with RXRalpha after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data