

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50395608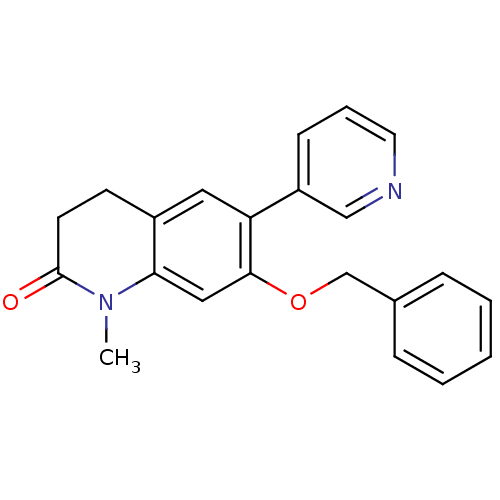 (CHEMBL2165321) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]-androstendione as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50395607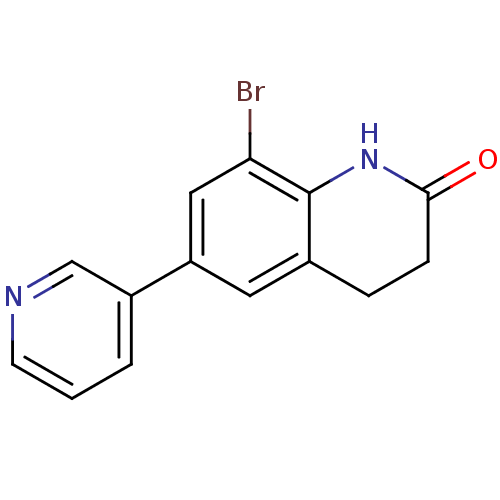 (CHEMBL2165322) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50318614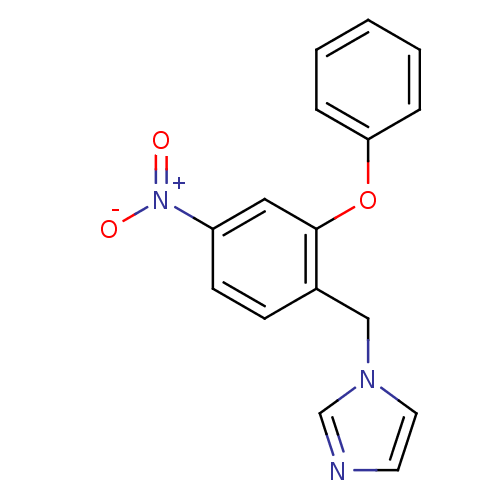 (1-(4-nitro-2-phenoxybenzyl)-1H-imidazole | CHEMBL1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]-androstendione as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50395612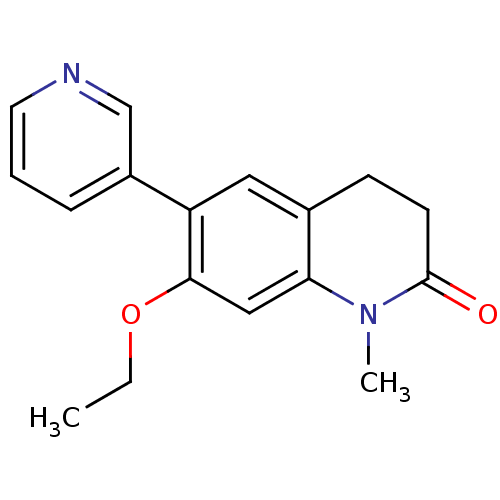 (CHEMBL2165317) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50395614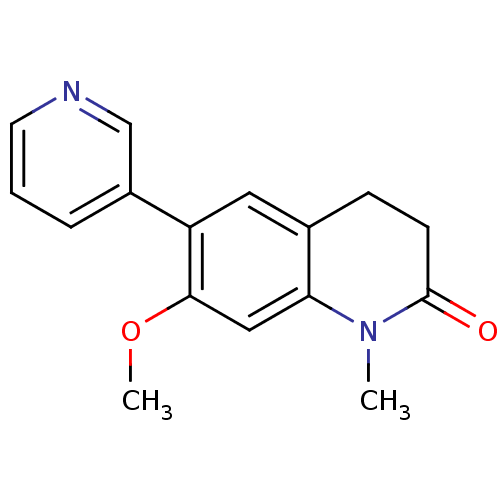 (CHEMBL2165315) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50395610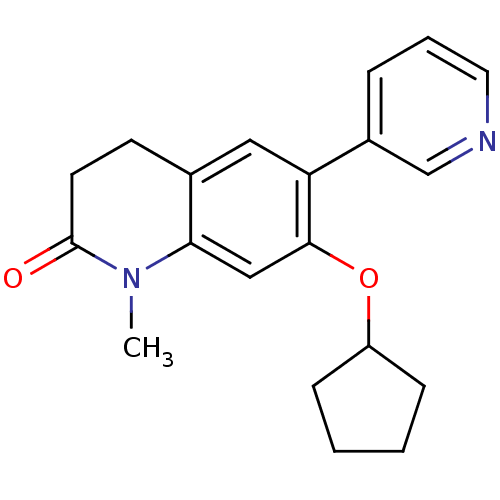 (CHEMBL2165319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]-androstendione as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50395609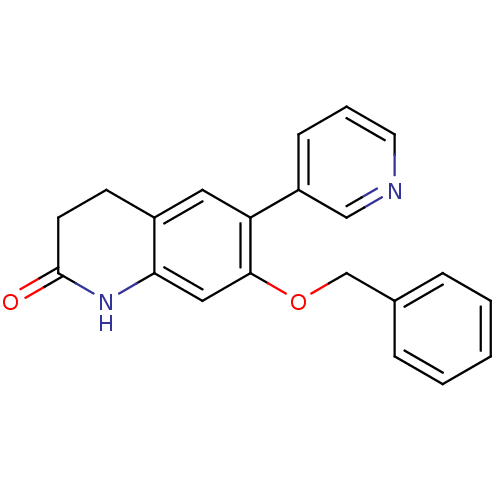 (CHEMBL2165320) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50395609 (CHEMBL2165320) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]-androstendione as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50395609 (CHEMBL2165320) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50395612 (CHEMBL2165317) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]-androstendione as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50395614 (CHEMBL2165315) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]-androstendione as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]-androstendione as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50395608 (CHEMBL2165321) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50395611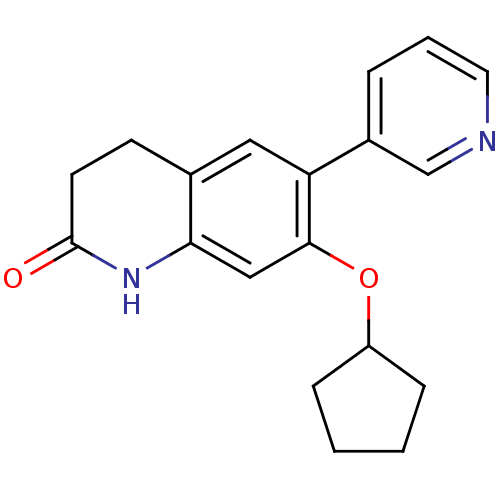 (CHEMBL2165318) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]-androstendione as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50395608 (CHEMBL2165321) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50395604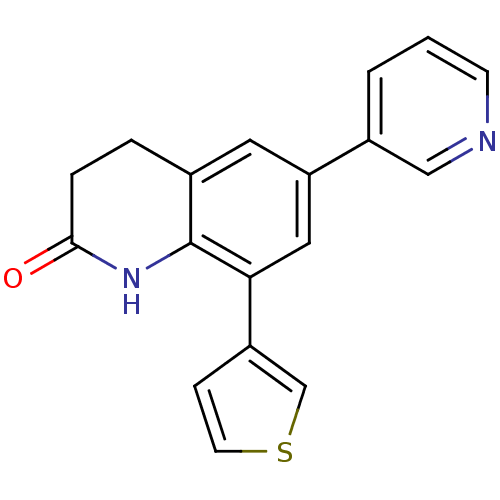 (CHEMBL2165325) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 208 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50395615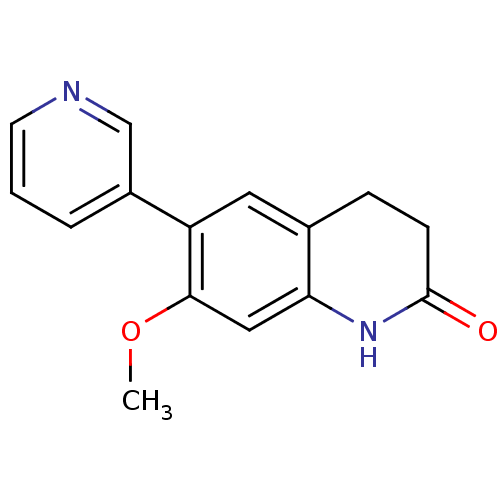 (CHEMBL2165314) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 268 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50395604 (CHEMBL2165325) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]-androstendione as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50395616 (CHEMBL2165313) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50395606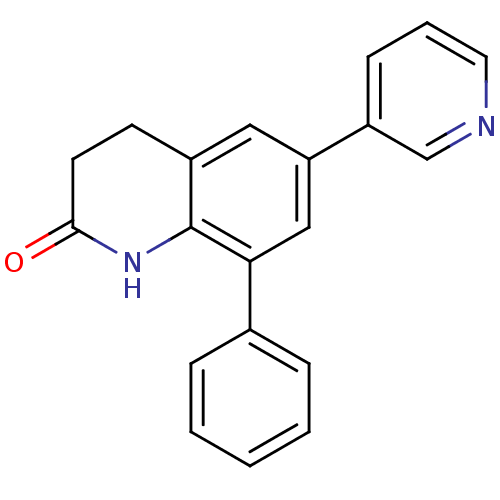 (CHEMBL2165323) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 394 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]-androstendione as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50273776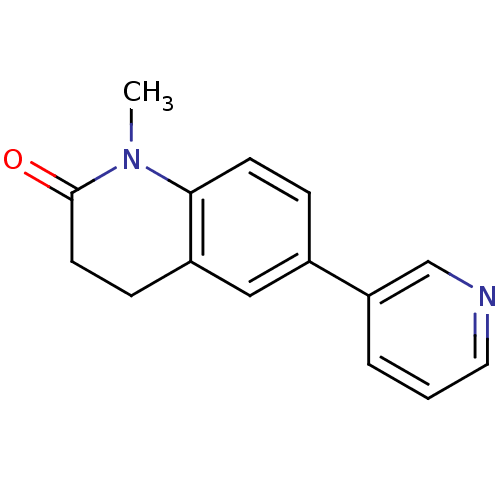 (1-Methyl-6-pyridin-3-yl-3,4-dihydroquinolin-2(1H)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 426 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]-androstendione as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50395615 (CHEMBL2165314) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 447 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]-androstendione as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50395613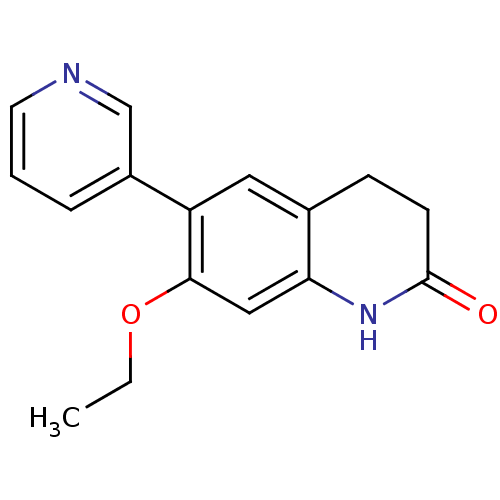 (CHEMBL2165316) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 488 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]-androstendione as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50395613 (CHEMBL2165316) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 674 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50395610 (CHEMBL2165319) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 759 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50395612 (CHEMBL2165317) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50395605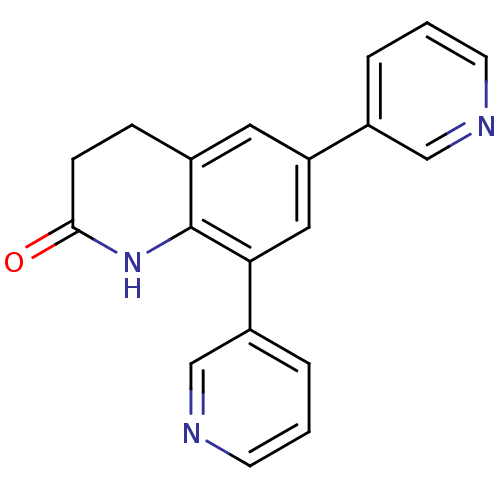 (CHEMBL2165324) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 954 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]-androstendione as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50395611 (CHEMBL2165318) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50395605 (CHEMBL2165324) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50395614 (CHEMBL2165315) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50395610 (CHEMBL2165319) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50395606 (CHEMBL2165323) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50395607 (CHEMBL2165322) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50395611 (CHEMBL2165318) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50395615 (CHEMBL2165314) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50395607 (CHEMBL2165322) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]-androstendione as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50395616 (CHEMBL2165313) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]-androstendione as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50395613 (CHEMBL2165316) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50395605 (CHEMBL2165324) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50395604 (CHEMBL2165325) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50000306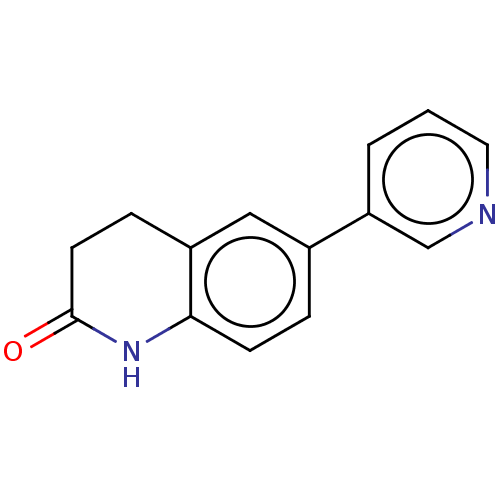 (6-Pyridin-3-yl-3,4-dihydro-1H-quinolin-2-one | 6-P...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes using [1beta-3H]-androstendione as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50395610 (CHEMBL2165319) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50395609 (CHEMBL2165320) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50395615 (CHEMBL2165314) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50395616 (CHEMBL2165313) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50395613 (CHEMBL2165316) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50395611 (CHEMBL2165318) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50395607 (CHEMBL2165322) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50395604 (CHEMBL2165325) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50395614 (CHEMBL2165315) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50395612 (CHEMBL2165317) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50395606 (CHEMBL2165323) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50395608 (CHEMBL2165321) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50395605 (CHEMBL2165324) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as a substrate | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50395616 (CHEMBL2165313) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50395606 (CHEMBL2165323) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in V79MZh cells using [14C]-11-deoxycorticosterone as substrate by HPTLC/phosphoimaging method | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50395614 (CHEMBL2165315) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of hepatic CYP3A4 | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50395612 (CHEMBL2165317) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of hepatic CYP3A4 | J Med Chem 55: 7080-9 (2012) Article DOI: 10.1021/jm3004637 BindingDB Entry DOI: 10.7270/Q2542PPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||