

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10867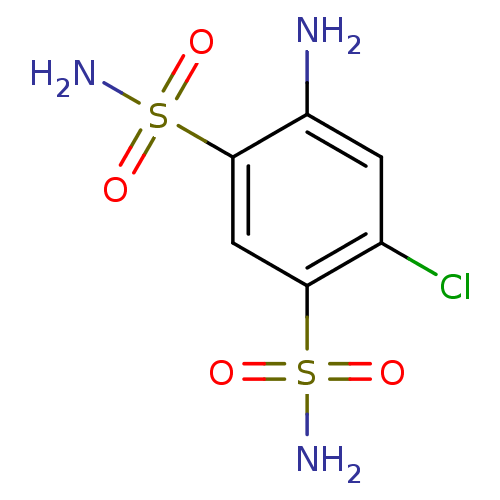 (4-amino-6-chlorobenzene-1,3-disulfonamide | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Cecen University Curated by ChEMBL | Assay Description Inhibition of human esterase activity of carbonic anhydrase 2 using 4-nitrophenylacetate as substrate after 3 mins by spectrophotometric analysis | Bioorg Med Chem 21: 1522-5 (2013) Article DOI: 10.1016/j.bmc.2012.08.018 BindingDB Entry DOI: 10.7270/Q2NG4S0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM29278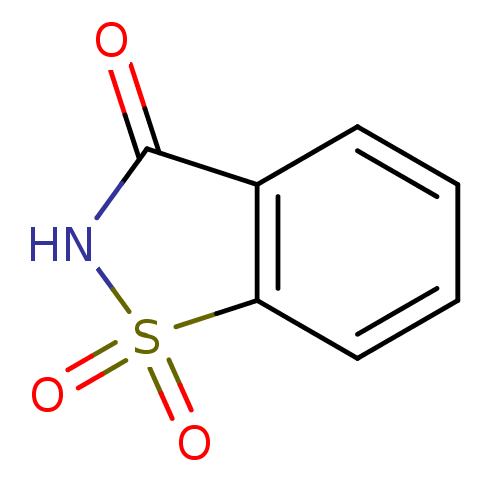 (CHEMBL310671 | Garantose | SAC | Saccharimide | Sa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Cecen University Curated by ChEMBL | Assay Description Inhibition of human esterase activity of carbonic anhydrase 9 using 4-nitrophenylacetate as substrate after 3 mins by spectrophotometric analysis | Bioorg Med Chem 21: 1522-5 (2013) Article DOI: 10.1016/j.bmc.2012.08.018 BindingDB Entry DOI: 10.7270/Q2NG4S0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10861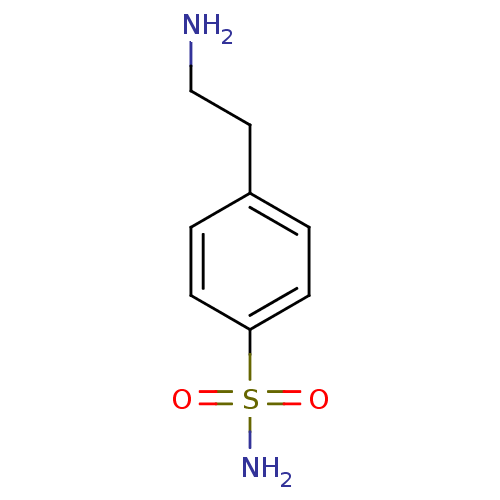 (4-(2-aminoethyl)benzene-1-sulfonamide | CHEMBL7087...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Cecen University Curated by ChEMBL | Assay Description Inhibition of human esterase activity of carbonic anhydrase 2 using 4-nitrophenylacetate as substrate after 3 mins by spectrophotometric analysis | Bioorg Med Chem 21: 1522-5 (2013) Article DOI: 10.1016/j.bmc.2012.08.018 BindingDB Entry DOI: 10.7270/Q2NG4S0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10859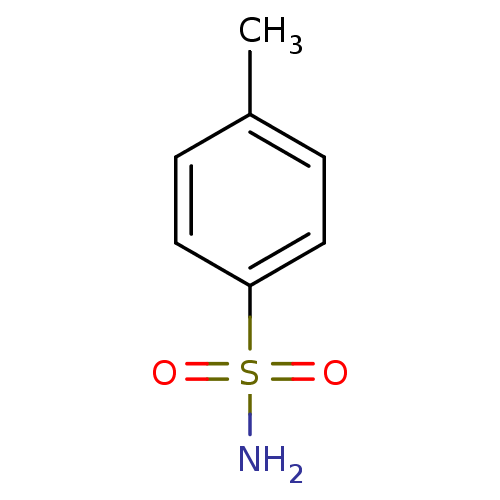 (4-methylbenzene-1-sulfonamide | CHEMBL574 | aromat...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Cecen University Curated by ChEMBL | Assay Description Inhibition of human esterase activity of carbonic anhydrase 2 using 4-nitrophenylacetate as substrate after 3 mins by spectrophotometric analysis | Bioorg Med Chem 21: 1522-5 (2013) Article DOI: 10.1016/j.bmc.2012.08.018 BindingDB Entry DOI: 10.7270/Q2NG4S0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12414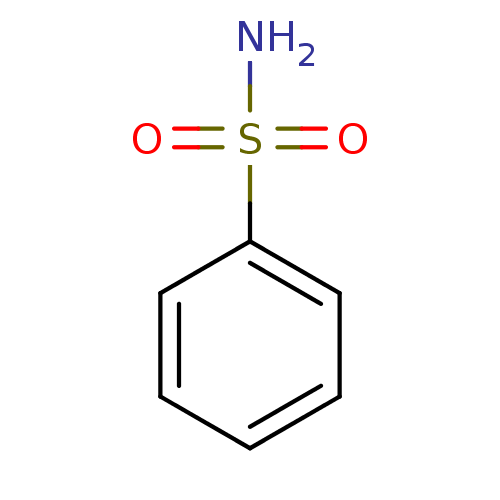 (CHEMBL27601 | benzenesulfonamide | hCA inhibitor, ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 5.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Cecen University Curated by ChEMBL | Assay Description Inhibition of human esterase activity of carbonic anhydrase 2 using 4-nitrophenylacetate as substrate after 3 mins by spectrophotometric analysis | Bioorg Med Chem 21: 1522-5 (2013) Article DOI: 10.1016/j.bmc.2012.08.018 BindingDB Entry DOI: 10.7270/Q2NG4S0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM29278 (CHEMBL310671 | Garantose | SAC | Saccharimide | Sa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 5.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Cecen University Curated by ChEMBL | Assay Description Inhibition of human esterase activity of carbonic anhydrase 2 using 4-nitrophenylacetate as substrate after 3 mins by spectrophotometric analysis | Bioorg Med Chem 21: 1522-5 (2013) Article DOI: 10.1016/j.bmc.2012.08.018 BindingDB Entry DOI: 10.7270/Q2NG4S0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10867 (4-amino-6-chlorobenzene-1,3-disulfonamide | CHEMBL...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Cecen University Curated by ChEMBL | Assay Description Inhibition of human esterase activity of carbonic anhydrase 1 using 4-nitrophenylacetate as substrate after 3 mins by spectrophotometric analysis | Bioorg Med Chem 21: 1522-5 (2013) Article DOI: 10.1016/j.bmc.2012.08.018 BindingDB Entry DOI: 10.7270/Q2NG4S0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10857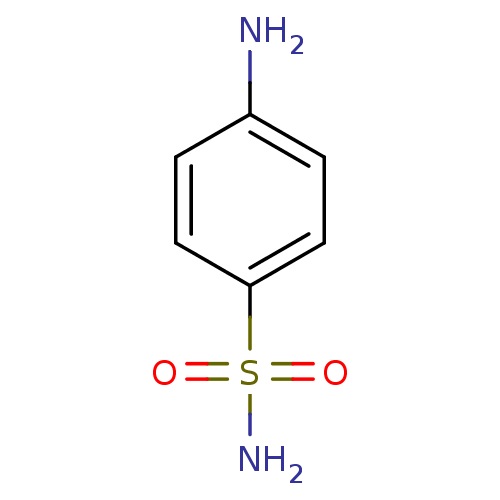 (4-aminobenzene-1-sulfonamide | CHEMBL21 | Sulfanil...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Cecen University Curated by ChEMBL | Assay Description Inhibition of human esterase activity of carbonic anhydrase 2 using 4-nitrophenylacetate as substrate after 3 mins by spectrophotometric analysis | Bioorg Med Chem 21: 1522-5 (2013) Article DOI: 10.1016/j.bmc.2012.08.018 BindingDB Entry DOI: 10.7270/Q2NG4S0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10860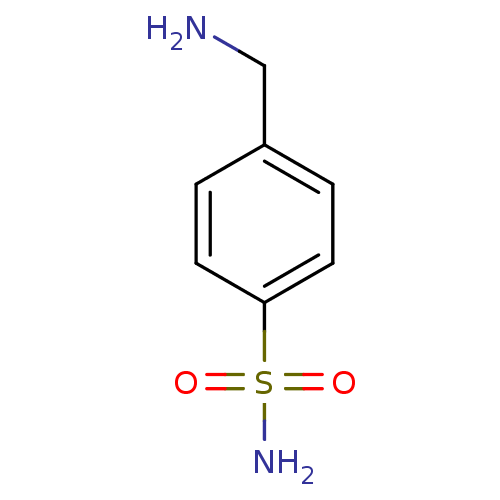 (4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Cecen University Curated by ChEMBL | Assay Description Inhibition of human esterase activity of carbonic anhydrase 2 using 4-nitrophenylacetate as substrate after 3 mins by spectrophotometric analysis | Bioorg Med Chem 21: 1522-5 (2013) Article DOI: 10.1016/j.bmc.2012.08.018 BindingDB Entry DOI: 10.7270/Q2NG4S0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM29278 (CHEMBL310671 | Garantose | SAC | Saccharimide | Sa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Cecen University Curated by ChEMBL | Assay Description Inhibition of human esterase activity of carbonic anhydrase 1 using 4-nitrophenylacetate as substrate after 3 mins by spectrophotometric analysis | Bioorg Med Chem 21: 1522-5 (2013) Article DOI: 10.1016/j.bmc.2012.08.018 BindingDB Entry DOI: 10.7270/Q2NG4S0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10861 (4-(2-aminoethyl)benzene-1-sulfonamide | CHEMBL7087...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Cecen University Curated by ChEMBL | Assay Description Inhibition of human esterase activity of carbonic anhydrase 1 using 4-nitrophenylacetate as substrate after 3 mins by spectrophotometric analysis | Bioorg Med Chem 21: 1522-5 (2013) Article DOI: 10.1016/j.bmc.2012.08.018 BindingDB Entry DOI: 10.7270/Q2NG4S0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10860 (4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Cecen University Curated by ChEMBL | Assay Description Inhibition of human esterase activity of carbonic anhydrase 1 using 4-nitrophenylacetate as substrate after 3 mins by spectrophotometric analysis | Bioorg Med Chem 21: 1522-5 (2013) Article DOI: 10.1016/j.bmc.2012.08.018 BindingDB Entry DOI: 10.7270/Q2NG4S0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10857 (4-aminobenzene-1-sulfonamide | CHEMBL21 | Sulfanil...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Cecen University Curated by ChEMBL | Assay Description Inhibition of human esterase activity of carbonic anhydrase 1 using 4-nitrophenylacetate as substrate after 3 mins by spectrophotometric analysis | Bioorg Med Chem 21: 1522-5 (2013) Article DOI: 10.1016/j.bmc.2012.08.018 BindingDB Entry DOI: 10.7270/Q2NG4S0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||