

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424427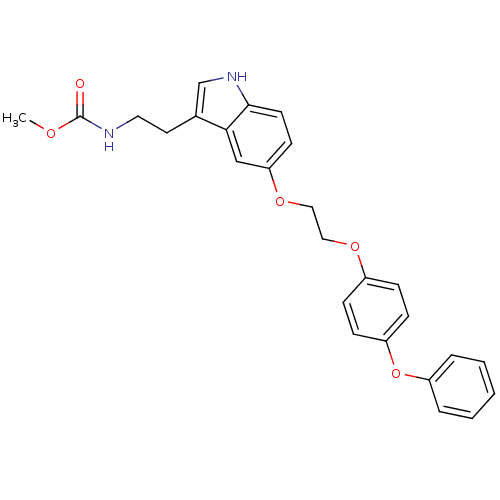 (CHEMBL2316064) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H aminopeptidase activity using Ala-p-nitroanilide as substrate incubated for 2 mins prior to substrate addition measured for... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM23738 (2-(1-benzyl-5-methoxy-2-methyl-1H-indol-3-yl)aceta...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 using 1,2-dimyristoyl-sn-glycero-3-phosphocholine as substrate after 10 mins by spectrop... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424432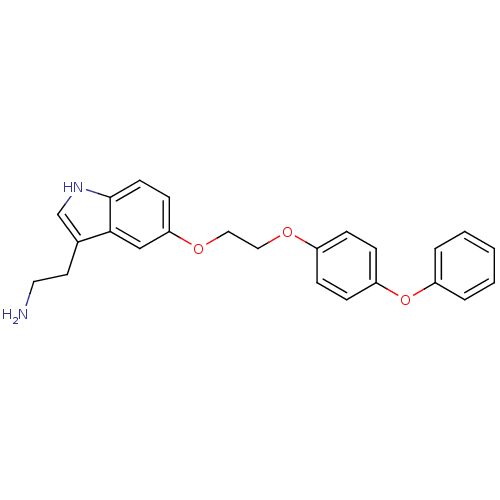 (CHEMBL2316059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H aminopeptidase activity using Ala-p-nitroanilide as substrate incubated for 2 mins prior to substrate addition measured for... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424434 (CHEMBL2316057) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H aminopeptidase activity using Ala-p-nitroanilide as substrate incubated for 2 mins prior to substrate addition measured for... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424427 (CHEMBL2316064) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424426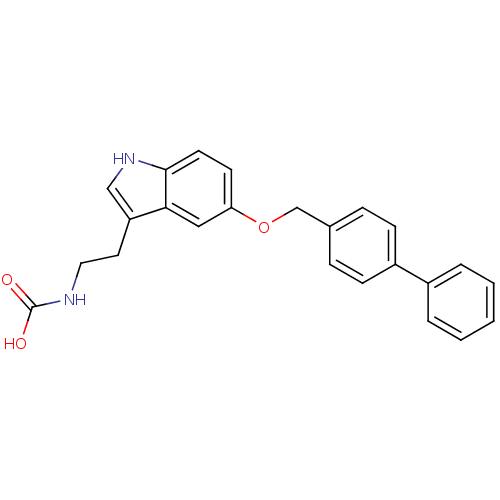 (CHEMBL2316065) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50424434 (CHEMBL2316057) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 using 1,2-dimyristoyl-sn-glycero-3-phosphocholine as substrate after 10 mins by spectrop... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424424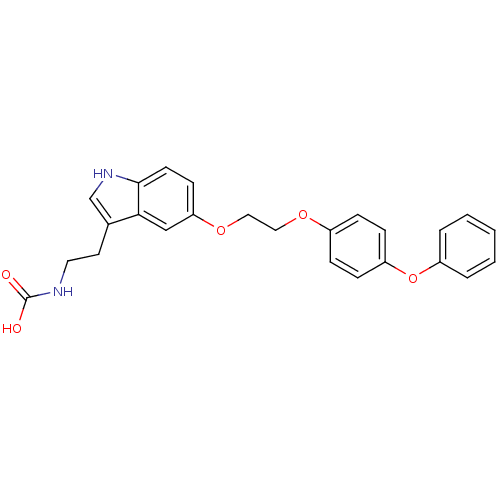 (CHEMBL2316067) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H aminopeptidase activity using Ala-p-nitroanilide as substrate incubated for 2 mins prior to substrate addition measured for... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50424431 (CHEMBL2316060) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 using 1,2-dimyristoyl-sn-glycero-3-phosphocholine as substrate after 10 mins by spectrop... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424429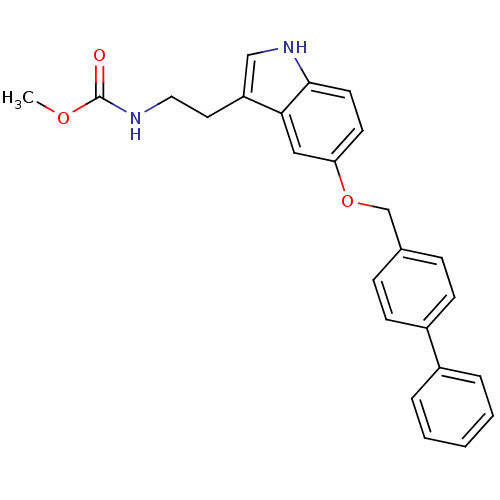 (CHEMBL2316062) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H aminopeptidase activity using Ala-p-nitroanilide as substrate incubated for 2 mins prior to substrate addition measured for... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424430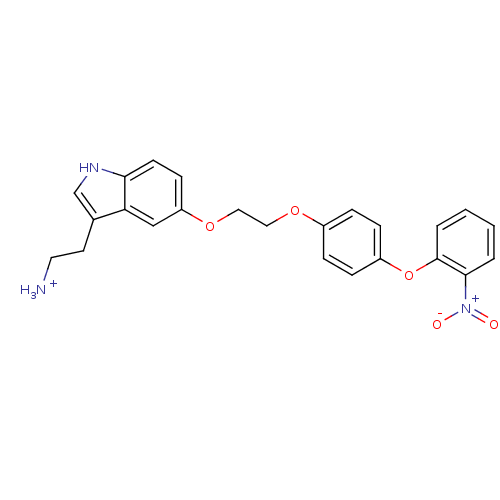 (CHEMBL2316061) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H aminopeptidase activity using Ala-p-nitroanilide as substrate incubated for 2 mins prior to substrate addition measured for... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424426 (CHEMBL2316065) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H aminopeptidase activity using Ala-p-nitroanilide as substrate incubated for 2 mins prior to substrate addition measured for... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424429 (CHEMBL2316062) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50424430 (CHEMBL2316061) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 using 1,2-dimyristoyl-sn-glycero-3-phosphocholine as substrate after 10 mins by spectrop... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424436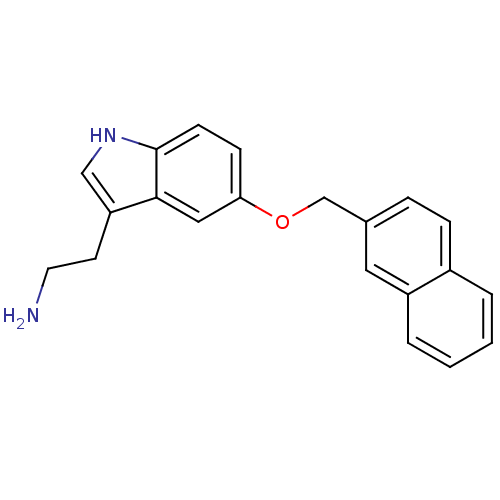 (CHEMBL2316069) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H aminopeptidase activity using Ala-p-nitroanilide as substrate incubated for 2 mins prior to substrate addition measured for... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424433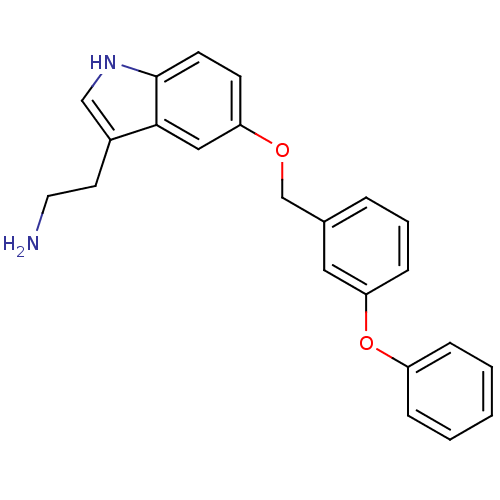 (CHEMBL2316058) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H aminopeptidase activity using Ala-p-nitroanilide as substrate incubated for 2 mins prior to substrate addition measured for... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50424427 (CHEMBL2316064) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 using 1,2-dimyristoyl-sn-glycero-3-phosphocholine as substrate after 10 mins by spectrop... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424428 (CHEMBL2316063) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H aminopeptidase activity using Ala-p-nitroanilide as substrate incubated for 2 mins prior to substrate addition measured for... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424425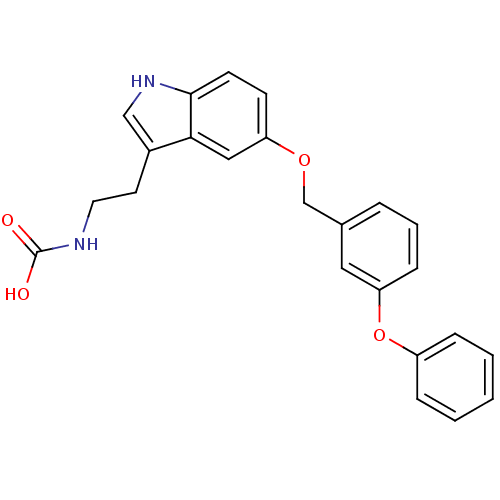 (CHEMBL2316066) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H aminopeptidase activity using Ala-p-nitroanilide as substrate incubated for 2 mins prior to substrate addition measured for... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50424432 (CHEMBL2316059) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 using 1,2-dimyristoyl-sn-glycero-3-phosphocholine as substrate after 10 mins by spectrop... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM82302 (5-BENZYLOXYTRYPTAMINE | 5-BZT | CAS_20776-45-8 | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H aminopeptidase activity using Ala-p-nitroanilide as substrate incubated for 2 mins prior to substrate addition measured for... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424432 (CHEMBL2316059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50424433 (CHEMBL2316058) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 using 1,2-dimyristoyl-sn-glycero-3-phosphocholine as substrate after 10 mins by spectrop... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424428 (CHEMBL2316063) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50247033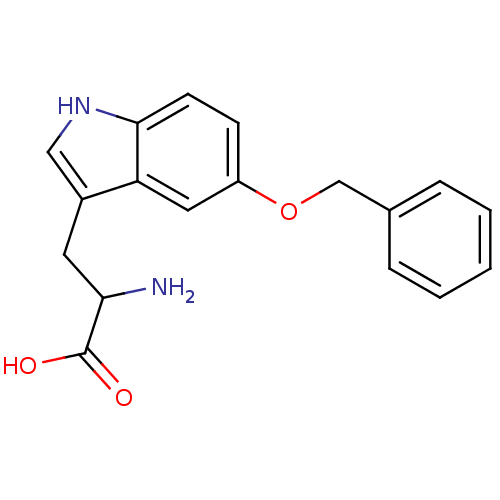 (2-amino-3-(5-(benzyloxy)-1H-indol-3-yl)propanoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H aminopeptidase activity using Ala-p-nitroanilide as substrate incubated for 2 mins prior to substrate addition measured for... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424434 (CHEMBL2316057) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50424428 (CHEMBL2316063) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 using 1,2-dimyristoyl-sn-glycero-3-phosphocholine as substrate after 10 mins by spectrop... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50424436 (CHEMBL2316069) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 using 1,2-dimyristoyl-sn-glycero-3-phosphocholine as substrate after 10 mins by spectrop... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50424435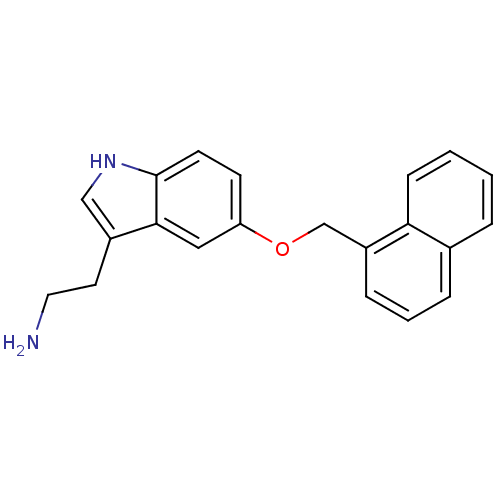 (CHEMBL2316056) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 using 1,2-dimyristoyl-sn-glycero-3-phosphocholine as substrate after 10 mins by spectrop... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424435 (CHEMBL2316056) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H aminopeptidase activity using Ala-p-nitroanilide as substrate incubated for 2 mins prior to substrate addition measured for... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50424429 (CHEMBL2316062) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 using 1,2-dimyristoyl-sn-glycero-3-phosphocholine as substrate after 10 mins by spectrop... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424431 (CHEMBL2316060) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H aminopeptidase activity using Ala-p-nitroanilide as substrate incubated for 2 mins prior to substrate addition measured for... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50247033 (2-amino-3-(5-(benzyloxy)-1H-indol-3-yl)propanoic a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 using 1,2-dimyristoyl-sn-glycero-3-phosphocholine as substrate after 10 mins by spectrop... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM82302 (5-BENZYLOXYTRYPTAMINE | 5-BZT | CAS_20776-45-8 | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 using 1,2-dimyristoyl-sn-glycero-3-phosphocholine as substrate after 10 mins by spectrop... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50424426 (CHEMBL2316065) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 using 1,2-dimyristoyl-sn-glycero-3-phosphocholine as substrate after 10 mins by spectrop... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50424425 (CHEMBL2316066) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 using 1,2-dimyristoyl-sn-glycero-3-phosphocholine as substrate after 10 mins by spectrop... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50424424 (CHEMBL2316067) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 using 1,2-dimyristoyl-sn-glycero-3-phosphocholine as substrate after 10 mins by spectrop... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424435 (CHEMBL2316056) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424430 (CHEMBL2316061) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424433 (CHEMBL2316058) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424425 (CHEMBL2316066) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424436 (CHEMBL2316069) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human nonpancreatic secretory phospholipase A2 using 1,2-dimyristoyl-sn-glycero-3-phosphocholine as substrate after 10 mins by spectrop... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM23738 (2-(1-benzyl-5-methoxy-2-methyl-1H-indol-3-yl)aceta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM23738 (2-(1-benzyl-5-methoxy-2-methyl-1H-indol-3-yl)aceta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H aminopeptidase activity using Ala-p-nitroanilide as substrate incubated for 2 mins prior to substrate addition measured for... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424424 (CHEMBL2316067) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM82302 (5-BENZYLOXYTRYPTAMINE | 5-BZT | CAS_20776-45-8 | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50424431 (CHEMBL2316060) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50247033 (2-amino-3-(5-(benzyloxy)-1H-indol-3-yl)propanoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... | Eur J Med Chem 59: 160-7 (2013) Article DOI: 10.1016/j.ejmech.2012.10.057 BindingDB Entry DOI: 10.7270/Q27P90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||