

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50449388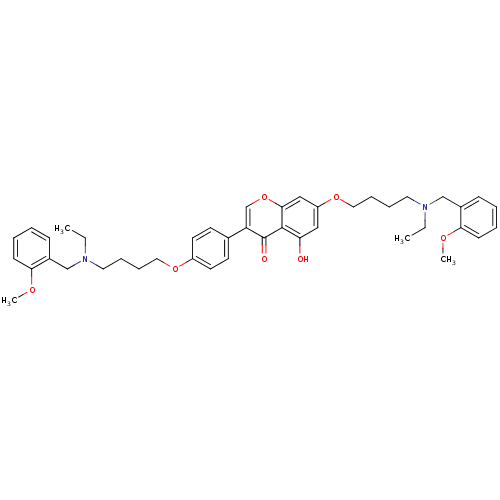 (CHEMBL3127003) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AchE using acetylthiocholine as substrate after 15 mins by Lineweaver-Burk plot analysis | Eur J Med Chem 76: 314-31 (2014) Article DOI: 10.1016/j.ejmech.2014.02.045 BindingDB Entry DOI: 10.7270/Q20R9QXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AchE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 76: 314-31 (2014) Article DOI: 10.1016/j.ejmech.2014.02.045 BindingDB Entry DOI: 10.7270/Q20R9QXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50449388 (CHEMBL3127003) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AchE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 76: 314-31 (2014) Article DOI: 10.1016/j.ejmech.2014.02.045 BindingDB Entry DOI: 10.7270/Q20R9QXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50449389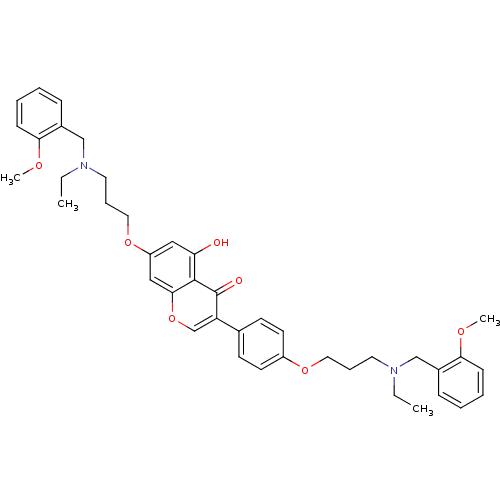 (CHEMBL3127001) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AchE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 76: 314-31 (2014) Article DOI: 10.1016/j.ejmech.2014.02.045 BindingDB Entry DOI: 10.7270/Q20R9QXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50449387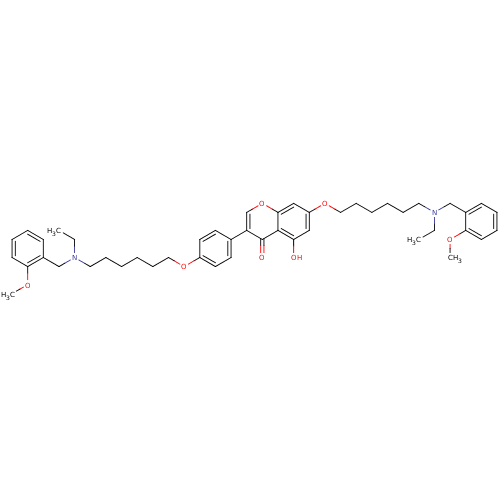 (CHEMBL3127004) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AchE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 76: 314-31 (2014) Article DOI: 10.1016/j.ejmech.2014.02.045 BindingDB Entry DOI: 10.7270/Q20R9QXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50449391 (CHEMBL3127006) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AchE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 76: 314-31 (2014) Article DOI: 10.1016/j.ejmech.2014.02.045 BindingDB Entry DOI: 10.7270/Q20R9QXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50449390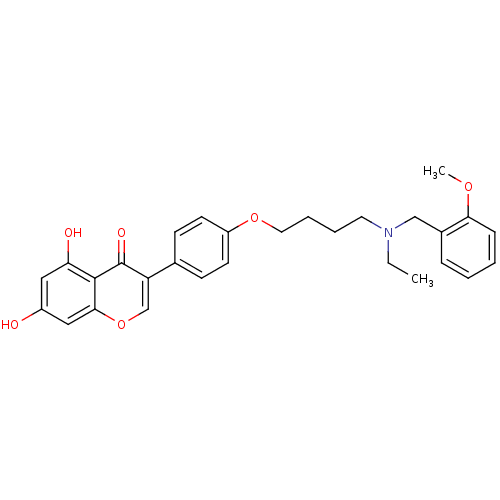 (CHEMBL3126996) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AchE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 76: 314-31 (2014) Article DOI: 10.1016/j.ejmech.2014.02.045 BindingDB Entry DOI: 10.7270/Q20R9QXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||