

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50400024 (CHEMBL2177719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50400020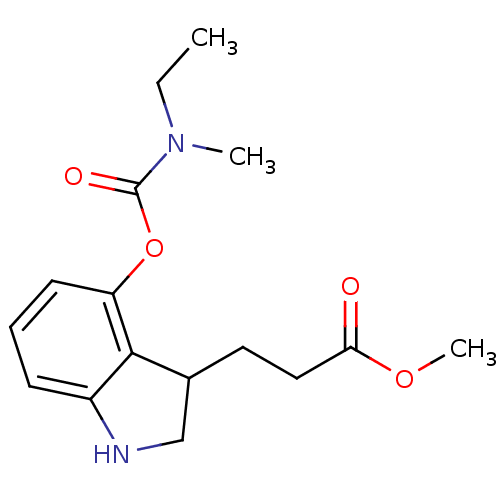 (CHEMBL2181475) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50013328 (CHEMBL3263347) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50400024 (CHEMBL2177719) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50400027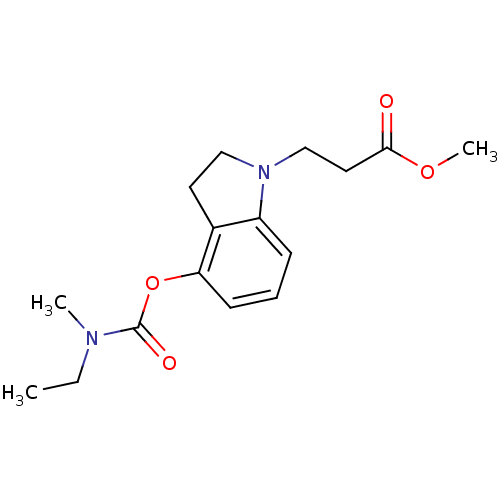 (CHEMBL2177716) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50013334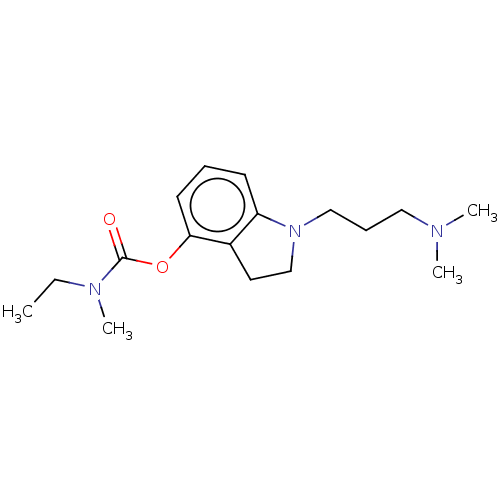 (CHEMBL3263351) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50013332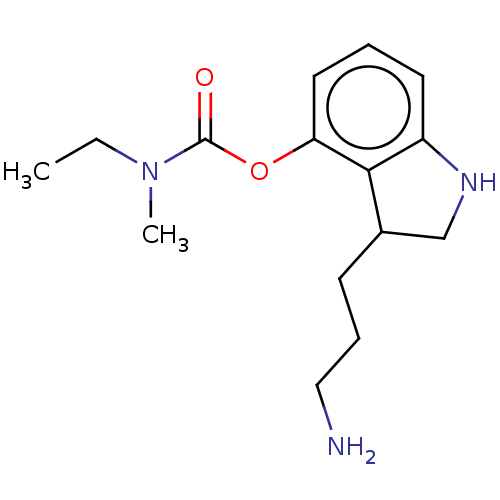 (CHEMBL3263349) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50013328 (CHEMBL3263347) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50013332 (CHEMBL3263349) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50013331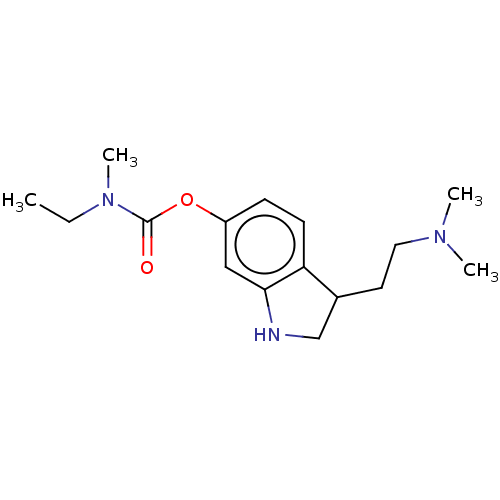 (CHEMBL3263348) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50013334 (CHEMBL3263351) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50400009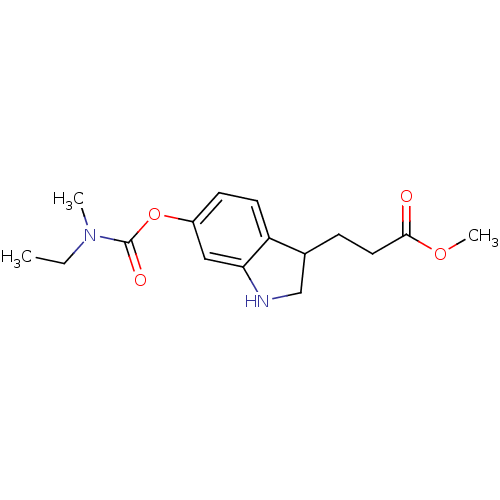 (CHEMBL2177708) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50013382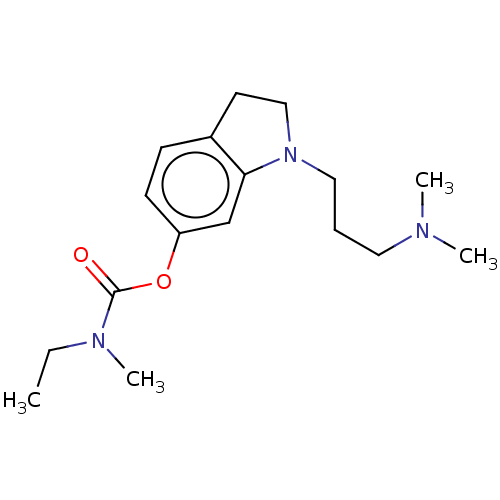 (CHEMBL3263352) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50013333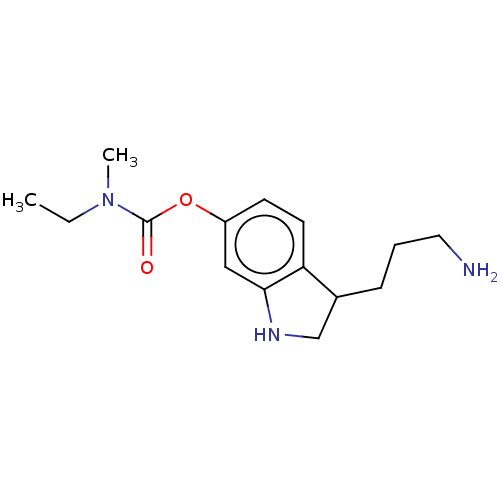 (CHEMBL3263350) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50400027 (CHEMBL2177716) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50400025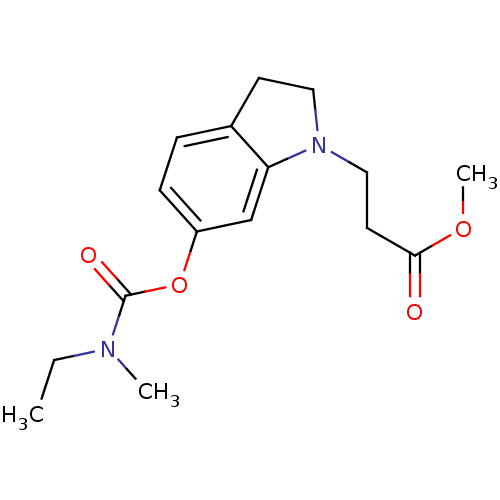 (CHEMBL2177718) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50400020 (CHEMBL2181475) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50013331 (CHEMBL3263348) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50013333 (CHEMBL3263350) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50013382 (CHEMBL3263352) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50400009 (CHEMBL2177708) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50400025 (CHEMBL2177718) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||