 Found 34 hits of Enzyme Inhibition Constant Data
Found 34 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | <6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | <62 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50090677
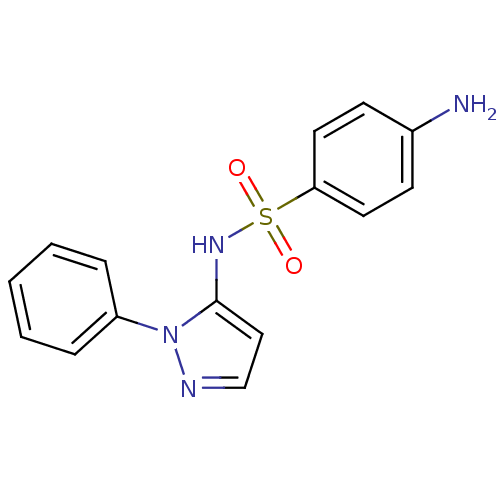
(4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...)Show InChI InChI=1S/C15H14N4O2S/c16-12-6-8-14(9-7-12)22(20,21)18-15-10-11-17-19(15)13-4-2-1-3-5-13/h1-11,18H,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50100556
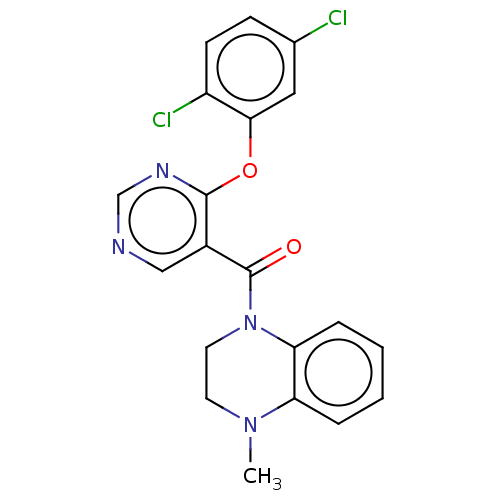
(CHEMBL3321845)Show SMILES CN1CCN(C(=O)c2cncnc2Oc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H16Cl2N4O2/c1-25-8-9-26(17-5-3-2-4-16(17)25)20(27)14-11-23-12-24-19(14)28-18-10-13(21)6-7-15(18)22/h2-7,10-12H,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50100557
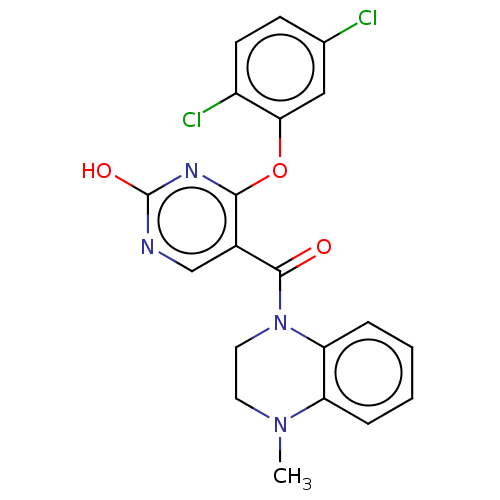
(CHEMBL3321846)Show SMILES CN1CCN(C(=O)c2cnc(O)nc2Oc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H16Cl2N4O3/c1-25-8-9-26(16-5-3-2-4-15(16)25)19(27)13-11-23-20(28)24-18(13)29-17-10-12(21)6-7-14(17)22/h2-7,10-11H,8-9H2,1H3,(H,23,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50100558

(CHEMBL3321850)Show SMILES CN1CCN(C(=O)C2(CC2)C(=O)Nc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H19Cl2N3O2/c1-24-10-11-25(17-5-3-2-4-16(17)24)19(27)20(8-9-20)18(26)23-15-12-13(21)6-7-14(15)22/h2-7,12H,8-11H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50100558

(CHEMBL3321850)Show SMILES CN1CCN(C(=O)C2(CC2)C(=O)Nc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H19Cl2N3O2/c1-24-10-11-25(17-5-3-2-4-16(17)24)19(27)20(8-9-20)18(26)23-15-12-13(21)6-7-14(15)22/h2-7,12H,8-11H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50100557

(CHEMBL3321846)Show SMILES CN1CCN(C(=O)c2cnc(O)nc2Oc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H16Cl2N4O3/c1-25-8-9-26(16-5-3-2-4-15(16)25)19(27)13-11-23-20(28)24-18(13)29-17-10-12(21)6-7-14(17)22/h2-7,10-11H,8-9H2,1H3,(H,23,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50100556

(CHEMBL3321845)Show SMILES CN1CCN(C(=O)c2cncnc2Oc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H16Cl2N4O2/c1-25-8-9-26(17-5-3-2-4-16(17)25)20(27)14-11-23-12-24-19(14)28-18-10-13(21)6-7-15(18)22/h2-7,10-12H,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50100558

(CHEMBL3321850)Show SMILES CN1CCN(C(=O)C2(CC2)C(=O)Nc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H19Cl2N3O2/c1-24-10-11-25(17-5-3-2-4-16(17)24)19(27)20(8-9-20)18(26)23-15-12-13(21)6-7-14(15)22/h2-7,12H,8-11H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50100556

(CHEMBL3321845)Show SMILES CN1CCN(C(=O)c2cncnc2Oc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H16Cl2N4O2/c1-25-8-9-26(17-5-3-2-4-16(17)25)20(27)14-11-23-12-24-19(14)28-18-10-13(21)6-7-15(18)22/h2-7,10-12H,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50240772

((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50236897
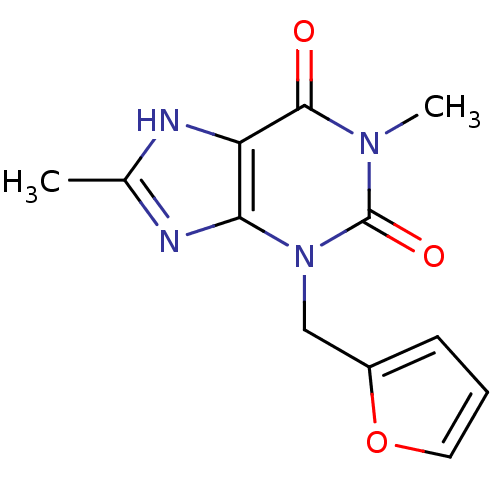
(3-(furan-2-ylmethyl)-1,8-dimethyl-1H-purine-2,6(3H...)Show InChI InChI=1S/C12H12N4O3/c1-7-13-9-10(14-7)16(6-8-4-3-5-19-8)12(18)15(2)11(9)17/h3-5H,6H2,1-2H3,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50100558

(CHEMBL3321850)Show SMILES CN1CCN(C(=O)C2(CC2)C(=O)Nc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H19Cl2N3O2/c1-24-10-11-25(17-5-3-2-4-16(17)24)19(27)20(8-9-20)18(26)23-15-12-13(21)6-7-14(15)22/h2-7,12H,8-11H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50100557

(CHEMBL3321846)Show SMILES CN1CCN(C(=O)c2cnc(O)nc2Oc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H16Cl2N4O3/c1-25-8-9-26(16-5-3-2-4-15(16)25)19(27)13-11-23-20(28)24-18(13)29-17-10-12(21)6-7-14(17)22/h2-7,10-11H,8-9H2,1H3,(H,23,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50100556

(CHEMBL3321845)Show SMILES CN1CCN(C(=O)c2cncnc2Oc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H16Cl2N4O2/c1-25-8-9-26(17-5-3-2-4-16(17)25)20(27)14-11-23-12-24-19(14)28-18-10-13(21)6-7-15(18)22/h2-7,10-12H,8-9H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50100557

(CHEMBL3321846)Show SMILES CN1CCN(C(=O)c2cnc(O)nc2Oc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H16Cl2N4O3/c1-25-8-9-26(16-5-3-2-4-15(16)25)19(27)13-11-23-20(28)24-18(13)29-17-10-12(21)6-7-14(17)22/h2-7,10-11H,8-9H2,1H3,(H,23,24,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50100556

(CHEMBL3321845)Show SMILES CN1CCN(C(=O)c2cncnc2Oc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H16Cl2N4O2/c1-25-8-9-26(17-5-3-2-4-16(17)25)20(27)14-11-23-12-24-19(14)28-18-10-13(21)6-7-15(18)22/h2-7,10-12H,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50100557

(CHEMBL3321846)Show SMILES CN1CCN(C(=O)c2cnc(O)nc2Oc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H16Cl2N4O3/c1-25-8-9-26(16-5-3-2-4-15(16)25)19(27)13-11-23-20(28)24-18(13)29-17-10-12(21)6-7-14(17)22/h2-7,10-11H,8-9H2,1H3,(H,23,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50100558

(CHEMBL3321850)Show SMILES CN1CCN(C(=O)C2(CC2)C(=O)Nc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H19Cl2N3O2/c1-24-10-11-25(17-5-3-2-4-16(17)24)19(27)20(8-9-20)18(26)23-15-12-13(21)6-7-14(15)22/h2-7,12H,8-11H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsome |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50100563
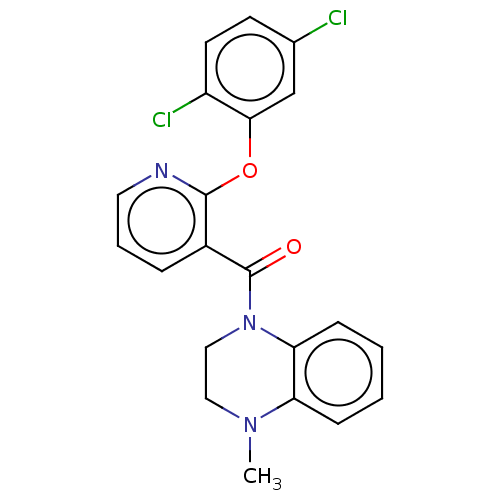
(CHEMBL3321841)Show SMILES CN1CCN(C(=O)c2cccnc2Oc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C21H17Cl2N3O2/c1-25-11-12-26(18-7-3-2-6-17(18)25)21(27)15-5-4-10-24-20(15)28-19-13-14(22)8-9-16(19)23/h2-10,13H,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in HEK293 cells assessed as cAMP level after 48 hrs by fluorescent assay |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50100567
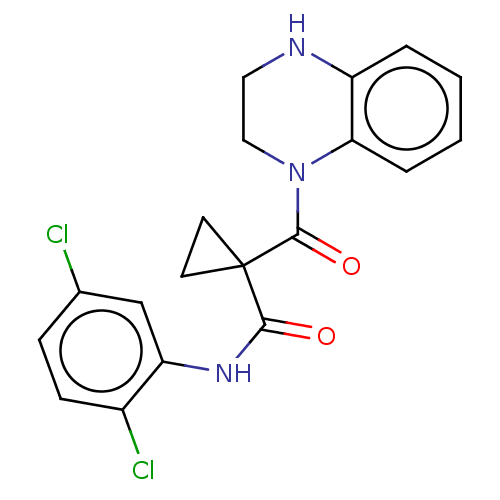
(CHEMBL3321849)Show SMILES Clc1ccc(Cl)c(NC(=O)C2(CC2)C(=O)N2CCNc3ccccc23)c1 Show InChI InChI=1S/C19H17Cl2N3O2/c20-12-5-6-13(21)15(11-12)23-17(25)19(7-8-19)18(26)24-10-9-22-14-3-1-2-4-16(14)24/h1-6,11,22H,7-10H2,(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 178 | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in HEK293 cells assessed as cAMP level after 48 hrs by fluorescent assay |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50100559

(CHEMBL3321844)Show SMILES CSc1ncc(C(=O)N2CCNc3ccccc23)c(Oc2cc(Cl)ccc2Cl)n1 Show InChI InChI=1S/C20H16Cl2N4O2S/c1-29-20-24-11-13(18(25-20)28-17-10-12(21)6-7-14(17)22)19(27)26-9-8-23-15-4-2-3-5-16(15)26/h2-7,10-11,23H,8-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in HEK293 cells assessed as cAMP level after 48 hrs by fluorescent assay |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50100562
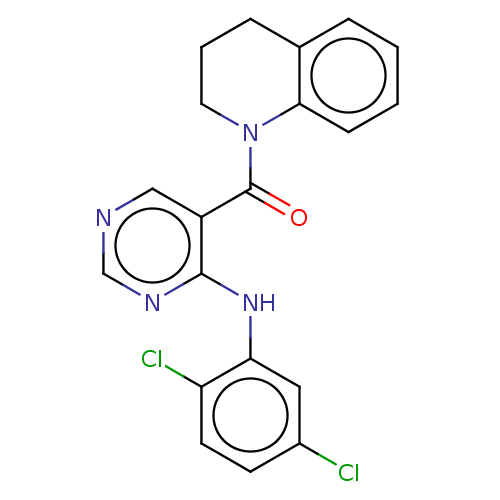
(CHEMBL3321842)Show SMILES Clc1ccc(Cl)c(Nc2ncncc2C(=O)N2CCCc3ccccc23)c1 Show InChI InChI=1S/C20H16Cl2N4O/c21-14-7-8-16(22)17(10-14)25-19-15(11-23-12-24-19)20(27)26-9-3-5-13-4-1-2-6-18(13)26/h1-2,4,6-8,10-12H,3,5,9H2,(H,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in HEK293 cells assessed as cAMP level after 48 hrs by fluorescent assay |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50100564

(CHEMBL3321847)Show InChI InChI=1S/C18H14Cl2N4O2/c1-24(15-5-3-2-4-14(15)21)18(25)12-9-22-10-23-17(12)26-16-8-11(19)6-7-13(16)20/h2-10H,21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in HEK293 cells assessed as cAMP level after 48 hrs by fluorescent assay |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50100568
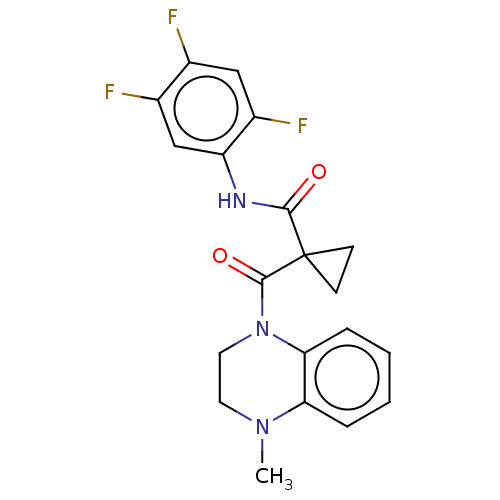
(CHEMBL3356914)Show SMILES CN1CCN(C(=O)C2(CC2)C(=O)Nc2cc(F)c(F)cc2F)c2ccccc12 Show InChI InChI=1S/C20H18F3N3O2/c1-25-8-9-26(17-5-3-2-4-16(17)25)19(28)20(6-7-20)18(27)24-15-11-13(22)12(21)10-14(15)23/h2-5,10-11H,6-9H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 122 | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in HEK293 cells assessed as cAMP level after 48 hrs by fluorescent assay |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50100560
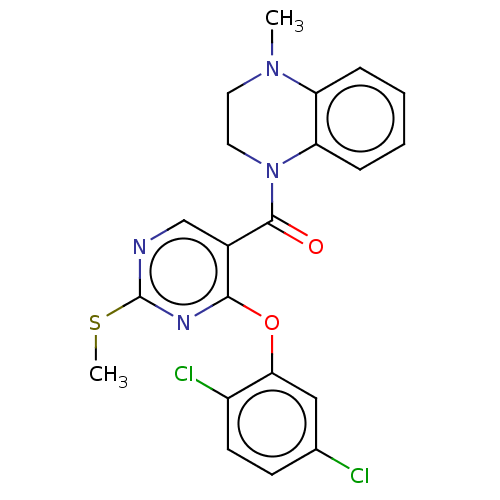
(CHEMBL3321843)Show SMILES CSc1ncc(C(=O)N2CCN(C)c3ccccc23)c(Oc2cc(Cl)ccc2Cl)n1 Show InChI InChI=1S/C21H18Cl2N4O2S/c1-26-9-10-27(17-6-4-3-5-16(17)26)20(28)14-12-24-21(30-2)25-19(14)29-18-11-13(22)7-8-15(18)23/h3-8,11-12H,9-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in HEK293 cells assessed as cAMP level after 48 hrs by fluorescent assay |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50100561
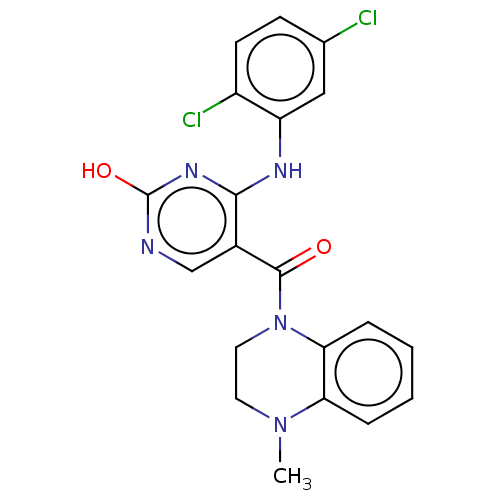
(CHEMBL3321758)Show SMILES CN1CCN(C(=O)c2cnc(O)nc2Nc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H17Cl2N5O2/c1-26-8-9-27(17-5-3-2-4-16(17)26)19(28)13-11-23-20(29)25-18(13)24-15-10-12(21)6-7-14(15)22/h2-7,10-11H,8-9H2,1H3,(H2,23,24,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in HEK293 cells assessed as cAMP level after 48 hrs by fluorescent assay |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50399945

(CHEMBL2181251)Show SMILES Clc1ccc(Cl)c(Oc2ncncc2C(=O)N2CCCc3ccccc23)c1 Show InChI InChI=1S/C20H15Cl2N3O2/c21-14-7-8-16(22)18(10-14)27-19-15(11-23-12-24-19)20(26)25-9-3-5-13-4-1-2-6-17(13)25/h1-2,4,6-8,10-12H,3,5,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in HEK293 cells assessed as cAMP level after 48 hrs by fluorescent assay |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50100556

(CHEMBL3321845)Show SMILES CN1CCN(C(=O)c2cncnc2Oc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H16Cl2N4O2/c1-25-8-9-26(17-5-3-2-4-16(17)25)20(27)14-11-23-12-24-19(14)28-18-10-13(21)6-7-15(18)22/h2-7,10-12H,8-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in HEK293 cells assessed as cAMP level after 48 hrs by fluorescent assay |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50100566

(CHEMBL3321848)Show SMILES CN(C(=O)C1(CC1)C(=O)N1CCCc2ccccc12)c1cc(Cl)ccc1Cl Show InChI InChI=1S/C21H20Cl2N2O2/c1-24(18-13-15(22)8-9-16(18)23)19(26)21(10-11-21)20(27)25-12-4-6-14-5-2-3-7-17(14)25/h2-3,5,7-9,13H,4,6,10-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in HEK293 cells assessed as cAMP level after 48 hrs by fluorescent assay |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50300199
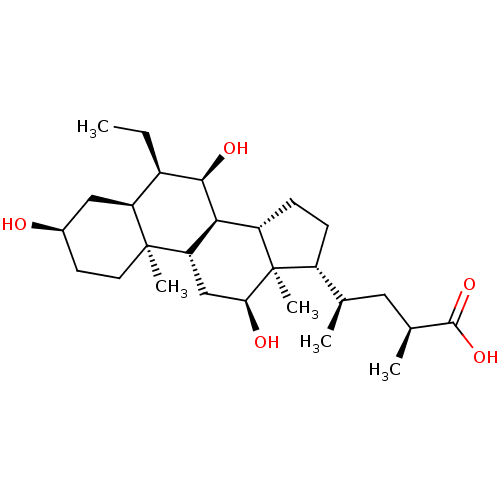
(6alpha-ethyl-23(S)-methyl-cholic acid | CHEMBL5676...)Show SMILES CC[C@H]1[C@@H](O)[C@H]2[C@@H]3CC[C@H]([C@H](C)C[C@H](C)C(O)=O)[C@@]3(C)[C@@H](O)C[C@@H]2[C@@]2(C)CC[C@@H](O)C[C@@H]12 |r| Show InChI InChI=1S/C27H46O5/c1-6-17-20-12-16(28)9-10-26(20,4)21-13-22(29)27(5)18(14(2)11-15(3)25(31)32)7-8-19(27)23(21)24(17)30/h14-24,28-30H,6-13H2,1-5H3,(H,31,32)/t14-,15+,16-,17-,18-,19+,20+,21+,22+,23+,24-,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 293 | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in HEK293 cells assessed as cAMP level after 48 hrs by fluorescent assay |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50100558

(CHEMBL3321850)Show SMILES CN1CCN(C(=O)C2(CC2)C(=O)Nc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H19Cl2N3O2/c1-24-10-11-25(17-5-3-2-4-16(17)24)19(27)20(8-9-20)18(26)23-15-12-13(21)6-7-14(15)22/h2-7,12H,8-11H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in HEK293 cells assessed as cAMP level after 48 hrs by fluorescent assay |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50100557

(CHEMBL3321846)Show SMILES CN1CCN(C(=O)c2cnc(O)nc2Oc2cc(Cl)ccc2Cl)c2ccccc12 Show InChI InChI=1S/C20H16Cl2N4O3/c1-25-8-9-26(16-5-3-2-4-15(16)25)19(27)13-11-23-20(28)24-18(13)29-17-10-12(21)6-7-14(17)22/h2-7,10-11H,8-9H2,1H3,(H,23,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a |
Chung-Ang University
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in HEK293 cells assessed as cAMP level after 48 hrs by fluorescent assay |
Bioorg Med Chem Lett 24: 4271-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.026
BindingDB Entry DOI: 10.7270/Q261122X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data