

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50100592 (CHEMBL3321889) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50100597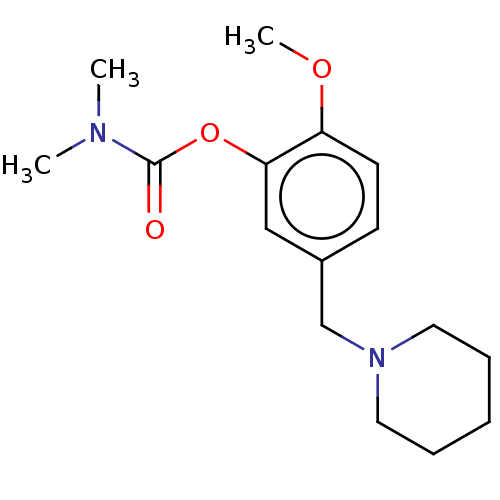 (CHEMBL3321877) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50100578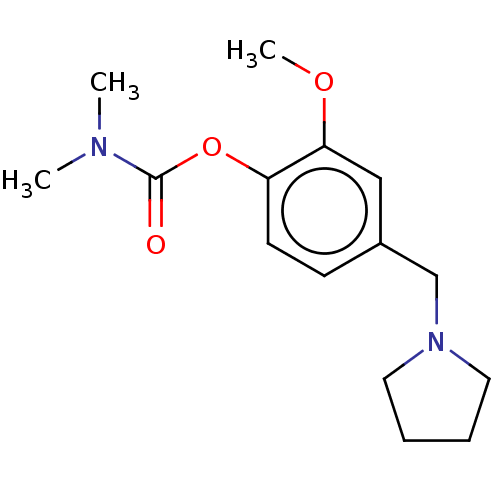 (CHEMBL3321887) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50100594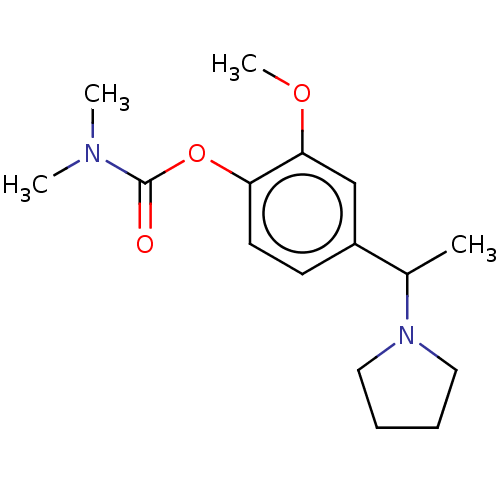 (CHEMBL3321874) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50100576 (CHEMBL3321885) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50100570 (CHEMBL3321879) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50100572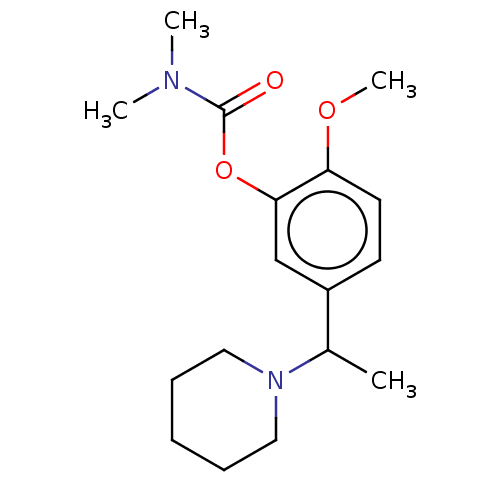 (CHEMBL3321881) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50100579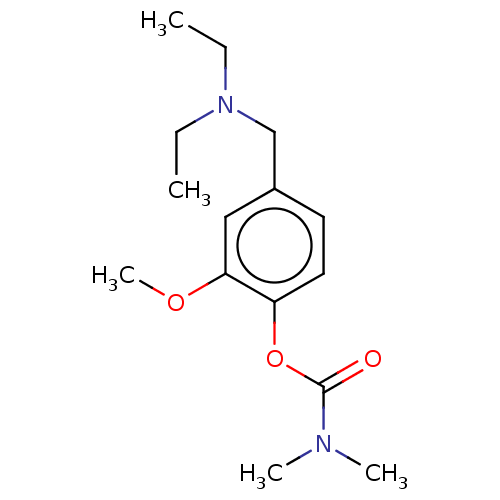 (CHEMBL3321888) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50100595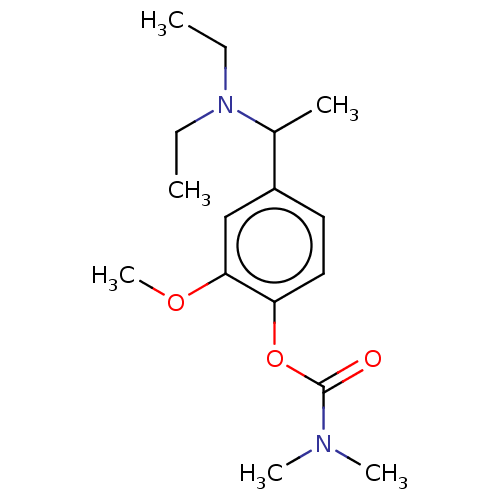 (CHEMBL3321875) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50100571 (CHEMBL3321880) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50100574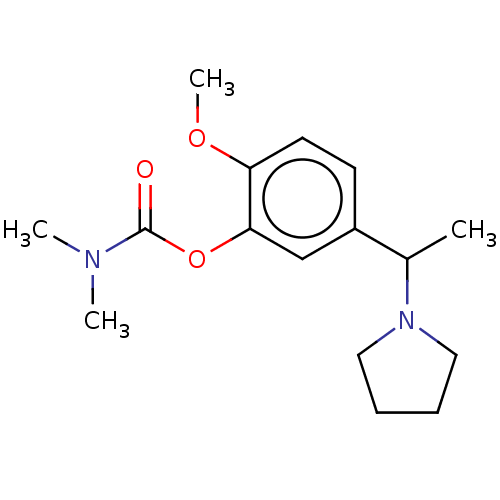 (CHEMBL3321883) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50100576 (CHEMBL3321885) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50100579 (CHEMBL3321888) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50100592 (CHEMBL3321889) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50100569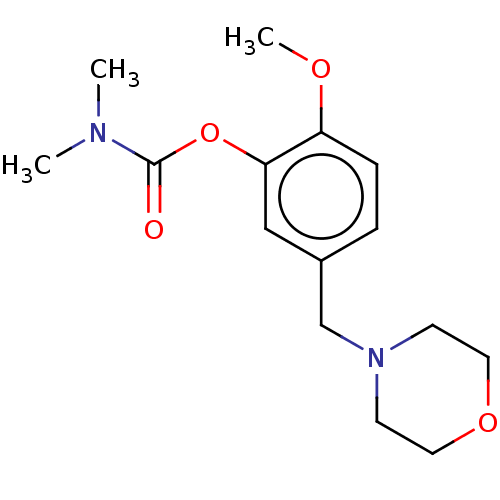 (CHEMBL3321878) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50100578 (CHEMBL3321887) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50100575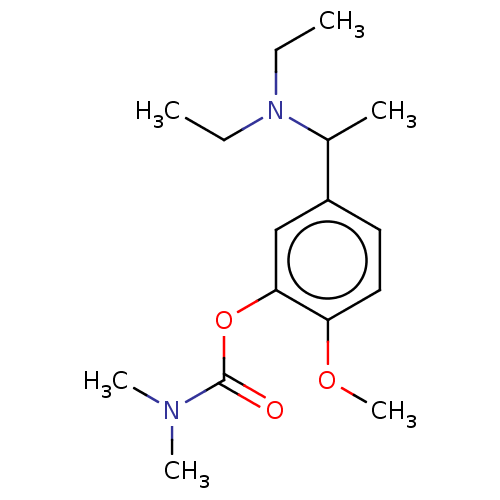 (CHEMBL3321884) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50100594 (CHEMBL3321874) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50100595 (CHEMBL3321875) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50100573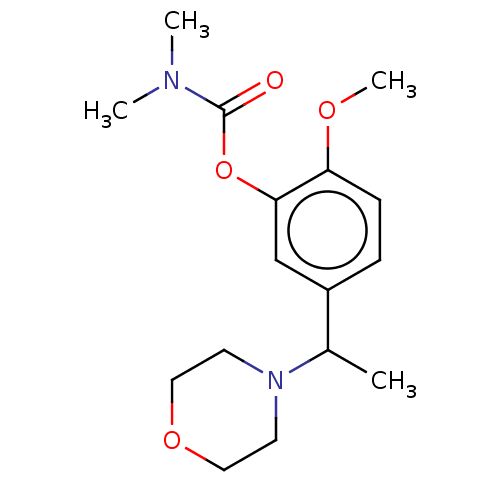 (CHEMBL3321882) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50100593 (CHEMBL3321890) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM10620 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50100577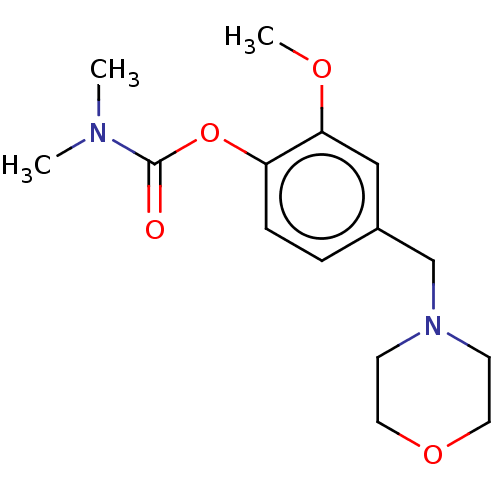 (CHEMBL3321886) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50100597 (CHEMBL3321877) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50100572 (CHEMBL3321881) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50100571 (CHEMBL3321880) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50100577 (CHEMBL3321886) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50100574 (CHEMBL3321883) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10620 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50100575 (CHEMBL3321884) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50100570 (CHEMBL3321879) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50100596 (CHEMBL3321876) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of BChE in rat serum using butyrylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50100593 (CHEMBL3321890) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50100569 (CHEMBL3321878) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50100596 (CHEMBL3321876) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50100573 (CHEMBL3321882) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex homogenates using acetylthiocholine iodide substrate incubated for 15 mins by UV spectroscopy based Ellman's method | Bioorg Med Chem 22: 4717-25 (2014) Article DOI: 10.1016/j.bmc.2014.07.009 BindingDB Entry DOI: 10.7270/Q22809B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||