 Found 46 hits of Enzyme Inhibition Constant Data
Found 46 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50393715

(CHEMBL2158994)Show InChI InChI=1S/C19H21NO2/c1-20(2)14-15-22-18-11-9-17(10-12-18)19(21)13-8-16-6-4-3-5-7-16/h3-13H,14-15H2,1-2H3/b13-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of AChE (unknown origin) incubated for 25 mins by Lineweaver-Burk plot analysis |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50393715

(CHEMBL2158994)Show InChI InChI=1S/C19H21NO2/c1-20(2)14-15-22-18-11-9-17(10-12-18)19(21)13-8-16-6-4-3-5-7-16/h3-13H,14-15H2,1-2H3/b13-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Competitive inhibition of AChE (unknown origin) incubated for 25 mins by Lineweaver-Burk plot analysis |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10620

((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50393715

(CHEMBL2158994)Show InChI InChI=1S/C19H21NO2/c1-20(2)14-15-22-18-11-9-17(10-12-18)19(21)13-8-16-6-4-3-5-7-16/h3-13H,14-15H2,1-2H3/b13-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099426

(CHEMBL3339001)Show InChI InChI=1S/C25H33NO2/c1-3-18-26(19-4-2)20-8-9-21-28-24-15-13-23(14-16-24)25(27)17-12-22-10-6-5-7-11-22/h5-7,10-17H,3-4,8-9,18-21H2,1-2H3/b17-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099419
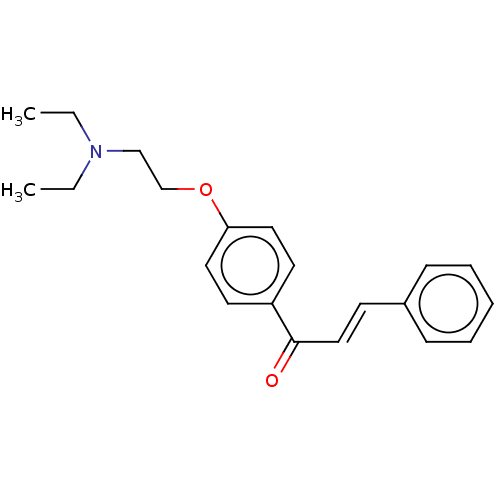
(CHEMBL3337471)Show InChI InChI=1S/C21H25NO2/c1-3-22(4-2)16-17-24-20-13-11-19(12-14-20)21(23)15-10-18-8-6-5-7-9-18/h5-15H,3-4,16-17H2,1-2H3/b15-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099426

(CHEMBL3339001)Show InChI InChI=1S/C25H33NO2/c1-3-18-26(19-4-2)20-8-9-21-28-24-15-13-23(14-16-24)25(27)17-12-22-10-6-5-7-11-22/h5-7,10-17H,3-4,8-9,18-21H2,1-2H3/b17-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099416

(CHEMBL3338992)Show InChI InChI=1S/C21H25NO2/c1-22(2)16-6-7-17-24-20-13-11-19(12-14-20)21(23)15-10-18-8-4-3-5-9-18/h3-5,8-15H,6-7,16-17H2,1-2H3/b15-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099425
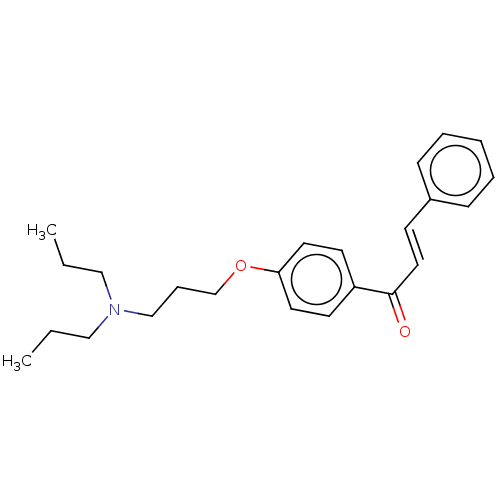
(CHEMBL3339000)Show InChI InChI=1S/C24H31NO2/c1-3-17-25(18-4-2)19-8-20-27-23-14-12-22(13-15-23)24(26)16-11-21-9-6-5-7-10-21/h5-7,9-16H,3-4,8,17-20H2,1-2H3/b16-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099421
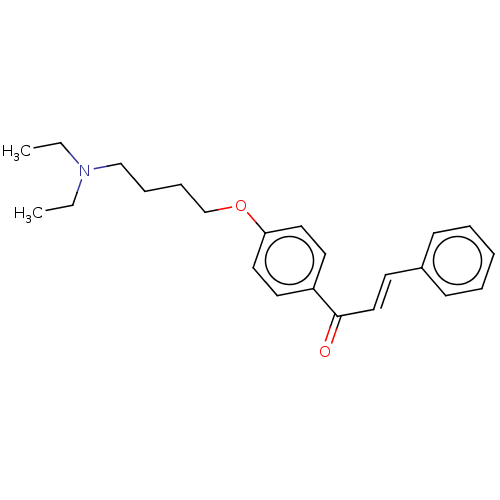
(CHEMBL3338996)Show InChI InChI=1S/C23H29NO2/c1-3-24(4-2)18-8-9-19-26-22-15-13-21(14-16-22)23(25)17-12-20-10-6-5-7-11-20/h5-7,10-17H,3-4,8-9,18-19H2,1-2H3/b17-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099418
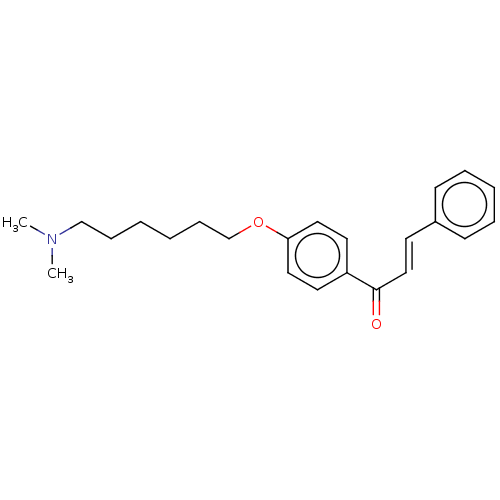
(CHEMBL3338994)Show InChI InChI=1S/C23H29NO2/c1-24(2)18-8-3-4-9-19-26-22-15-13-21(14-16-22)23(25)17-12-20-10-6-5-7-11-20/h5-7,10-17H,3-4,8-9,18-19H2,1-2H3/b17-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099414
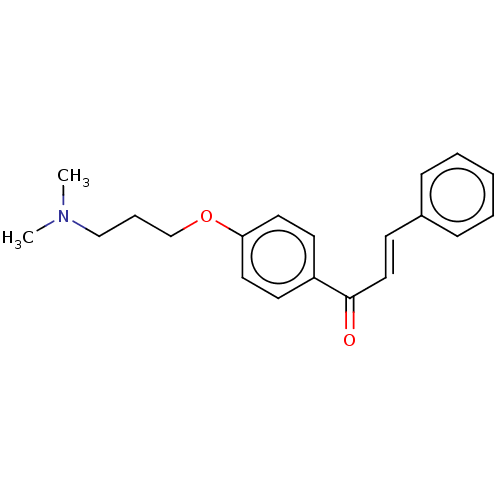
(CHEMBL3338991)Show InChI InChI=1S/C20H23NO2/c1-21(2)15-6-16-23-19-12-10-18(11-13-19)20(22)14-9-17-7-4-3-5-8-17/h3-5,7-14H,6,15-16H2,1-2H3/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10620

((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099416

(CHEMBL3338992)Show InChI InChI=1S/C21H25NO2/c1-22(2)16-6-7-17-24-20-13-11-19(12-14-20)21(23)15-10-18-8-4-3-5-9-18/h3-5,8-15H,6-7,16-17H2,1-2H3/b15-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099417

(CHEMBL3338993)Show InChI InChI=1S/C22H27NO2/c1-23(2)17-7-4-8-18-25-21-14-12-20(13-15-21)22(24)16-11-19-9-5-3-6-10-19/h3,5-6,9-16H,4,7-8,17-18H2,1-2H3/b16-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099418

(CHEMBL3338994)Show InChI InChI=1S/C23H29NO2/c1-24(2)18-8-3-4-9-19-26-22-15-13-21(14-16-22)23(25)17-12-20-10-6-5-7-11-20/h5-7,10-17H,3-4,8-9,18-19H2,1-2H3/b17-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099417

(CHEMBL3338993)Show InChI InChI=1S/C22H27NO2/c1-23(2)17-7-4-8-18-25-21-14-12-20(13-15-21)22(24)16-11-19-9-5-3-6-10-19/h3,5-6,9-16H,4,7-8,17-18H2,1-2H3/b16-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099423

(CHEMBL3338998)Show InChI InChI=1S/C25H33NO2/c1-3-26(4-2)20-10-5-6-11-21-28-24-17-15-23(16-18-24)25(27)19-14-22-12-8-7-9-13-22/h7-9,12-19H,3-6,10-11,20-21H2,1-2H3/b19-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099420
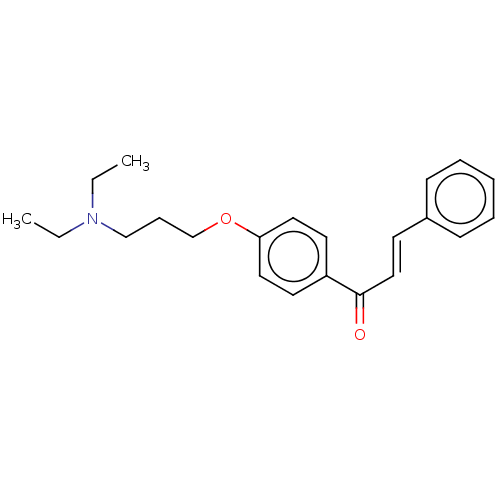
(CHEMBL3338995)Show InChI InChI=1S/C22H27NO2/c1-3-23(4-2)17-8-18-25-21-14-12-20(13-15-21)22(24)16-11-19-9-6-5-7-10-19/h5-7,9-16H,3-4,8,17-18H2,1-2H3/b16-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50393715

(CHEMBL2158994)Show InChI InChI=1S/C19H21NO2/c1-20(2)14-15-22-18-11-9-17(10-12-18)19(21)13-8-16-6-4-3-5-7-16/h3-13H,14-15H2,1-2H3/b13-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099431

(CHEMBL3339006)Show SMILES CCCCN(CCCC)CCCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C27H37NO2/c1-3-5-20-28(21-6-4-2)22-10-11-23-30-26-17-15-25(16-18-26)27(29)19-14-24-12-8-7-9-13-24/h7-9,12-19H,3-6,10-11,20-23H2,1-2H3/b19-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099420

(CHEMBL3338995)Show InChI InChI=1S/C22H27NO2/c1-3-23(4-2)17-8-18-25-21-14-12-20(13-15-21)22(24)16-11-19-9-6-5-7-10-19/h5-7,9-16H,3-4,8,17-18H2,1-2H3/b16-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099421

(CHEMBL3338996)Show InChI InChI=1S/C23H29NO2/c1-3-24(4-2)18-8-9-19-26-22-15-13-21(14-16-22)23(25)17-12-20-10-6-5-7-11-20/h5-7,10-17H,3-4,8-9,18-19H2,1-2H3/b17-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099425

(CHEMBL3339000)Show InChI InChI=1S/C24H31NO2/c1-3-17-25(18-4-2)19-8-20-27-23-14-12-22(13-15-23)24(26)16-11-21-9-6-5-7-10-21/h5-7,9-16H,3-4,8,17-20H2,1-2H3/b16-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099422

(CHEMBL3338997)Show InChI InChI=1S/C24H31NO2/c1-3-25(4-2)19-9-6-10-20-27-23-16-14-22(15-17-23)24(26)18-13-21-11-7-5-8-12-21/h5,7-8,11-18H,3-4,6,9-10,19-20H2,1-2H3/b18-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099427

(CHEMBL3339002)Show SMILES CCCN(CCC)CCCCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C26H35NO2/c1-3-19-27(20-4-2)21-9-6-10-22-29-25-16-14-24(15-17-25)26(28)18-13-23-11-7-5-8-12-23/h5,7-8,11-18H,3-4,6,9-10,19-22H2,1-2H3/b18-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099419

(CHEMBL3337471)Show InChI InChI=1S/C21H25NO2/c1-3-22(4-2)16-17-24-20-13-11-19(12-14-20)21(23)15-10-18-8-6-5-7-9-18/h5-15H,3-4,16-17H2,1-2H3/b15-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099414

(CHEMBL3338991)Show InChI InChI=1S/C20H23NO2/c1-21(2)15-6-16-23-19-12-10-18(11-13-19)20(22)14-9-17-7-4-3-5-8-17/h3-5,7-14H,6,15-16H2,1-2H3/b14-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099422

(CHEMBL3338997)Show InChI InChI=1S/C24H31NO2/c1-3-25(4-2)19-9-6-10-20-27-23-16-14-22(15-17-23)24(26)18-13-21-11-7-5-8-12-21/h5,7-8,11-18H,3-4,6,9-10,19-20H2,1-2H3/b18-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099427

(CHEMBL3339002)Show SMILES CCCN(CCC)CCCCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C26H35NO2/c1-3-19-27(20-4-2)21-9-6-10-22-29-25-16-14-24(15-17-25)26(28)18-13-23-11-7-5-8-12-23/h5,7-8,11-18H,3-4,6,9-10,19-22H2,1-2H3/b18-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099428

(CHEMBL3339003)Show SMILES CCCN(CCC)CCCCCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C27H37NO2/c1-3-20-28(21-4-2)22-10-5-6-11-23-30-26-17-15-25(16-18-26)27(29)19-14-24-12-8-7-9-13-24/h7-9,12-19H,3-6,10-11,20-23H2,1-2H3/b19-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099431

(CHEMBL3339006)Show SMILES CCCCN(CCCC)CCCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C27H37NO2/c1-3-5-20-28(21-6-4-2)22-10-11-23-30-26-17-15-25(16-18-26)27(29)19-14-24-12-8-7-9-13-24/h7-9,12-19H,3-6,10-11,20-23H2,1-2H3/b19-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099428

(CHEMBL3339003)Show SMILES CCCN(CCC)CCCCCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C27H37NO2/c1-3-20-28(21-4-2)22-10-5-6-11-23-30-26-17-15-25(16-18-26)27(29)19-14-24-12-8-7-9-13-24/h7-9,12-19H,3-6,10-11,20-23H2,1-2H3/b19-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099423

(CHEMBL3338998)Show InChI InChI=1S/C25H33NO2/c1-3-26(4-2)20-10-5-6-11-21-28-24-17-15-23(16-18-24)25(27)19-14-22-12-8-7-9-13-22/h7-9,12-19H,3-6,10-11,20-21H2,1-2H3/b19-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099424
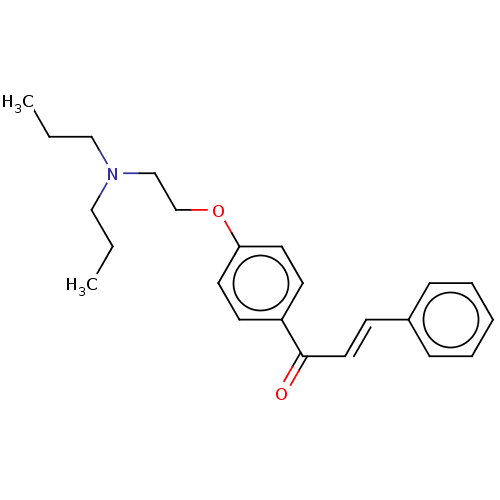
(CHEMBL3338999)Show InChI InChI=1S/C23H29NO2/c1-3-16-24(17-4-2)18-19-26-22-13-11-21(12-14-22)23(25)15-10-20-8-6-5-7-9-20/h5-15H,3-4,16-19H2,1-2H3/b15-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099424

(CHEMBL3338999)Show InChI InChI=1S/C23H29NO2/c1-3-16-24(17-4-2)18-19-26-22-13-11-21(12-14-22)23(25)15-10-20-8-6-5-7-9-20/h5-15H,3-4,16-19H2,1-2H3/b15-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099430
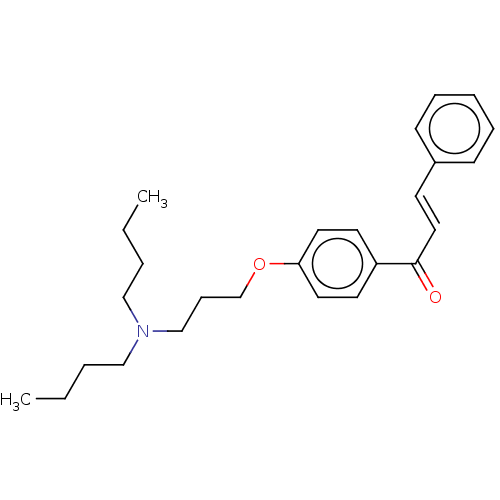
(CHEMBL3339005)Show SMILES CCCCN(CCCC)CCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C26H35NO2/c1-3-5-19-27(20-6-4-2)21-10-22-29-25-16-14-24(15-17-25)26(28)18-13-23-11-8-7-9-12-23/h7-9,11-18H,3-6,10,19-22H2,1-2H3/b18-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099433
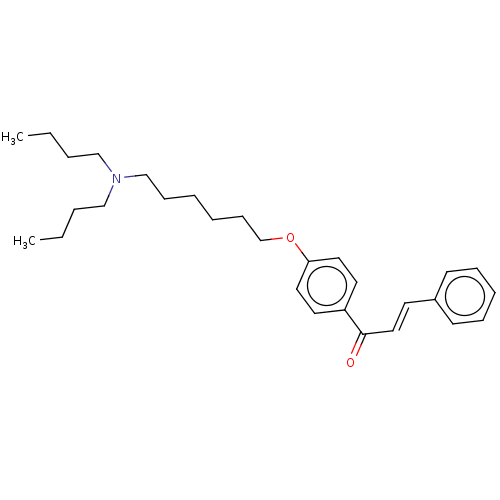
(CHEMBL3339008)Show SMILES CCCCN(CCCC)CCCCCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C29H41NO2/c1-3-5-22-30(23-6-4-2)24-12-7-8-13-25-32-28-19-17-27(18-20-28)29(31)21-16-26-14-10-9-11-15-26/h9-11,14-21H,3-8,12-13,22-25H2,1-2H3/b21-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099432
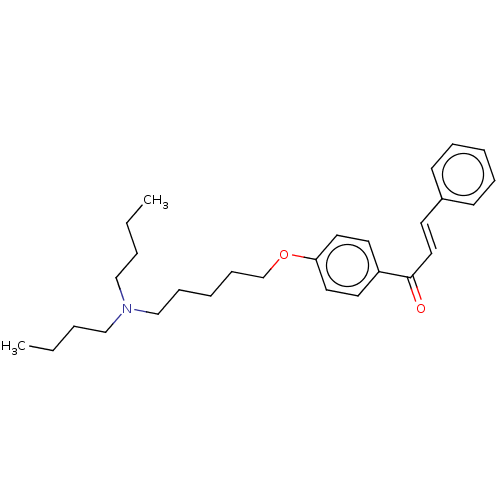
(CHEMBL3339007)Show SMILES CCCCN(CCCC)CCCCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C28H39NO2/c1-3-5-21-29(22-6-4-2)23-11-8-12-24-31-27-18-16-26(17-19-27)28(30)20-15-25-13-9-7-10-14-25/h7,9-10,13-20H,3-6,8,11-12,21-24H2,1-2H3/b20-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099432

(CHEMBL3339007)Show SMILES CCCCN(CCCC)CCCCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C28H39NO2/c1-3-5-21-29(22-6-4-2)23-11-8-12-24-31-27-18-16-26(17-19-27)28(30)20-15-25-13-9-7-10-14-25/h7,9-10,13-20H,3-6,8,11-12,21-24H2,1-2H3/b20-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099430

(CHEMBL3339005)Show SMILES CCCCN(CCCC)CCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C26H35NO2/c1-3-5-19-27(20-6-4-2)21-10-22-29-25-16-14-24(15-17-25)26(28)18-13-23-11-8-7-9-12-23/h7-9,11-18H,3-6,10,19-22H2,1-2H3/b18-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099433

(CHEMBL3339008)Show SMILES CCCCN(CCCC)CCCCCCOc1ccc(cc1)C(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C29H41NO2/c1-3-5-22-30(23-6-4-2)24-12-7-8-13-25-32-28-19-17-27(18-20-28)29(31)21-16-26-14-10-9-11-15-26/h9-11,14-21H,3-8,12-13,22-25H2,1-2H3/b21-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50099429
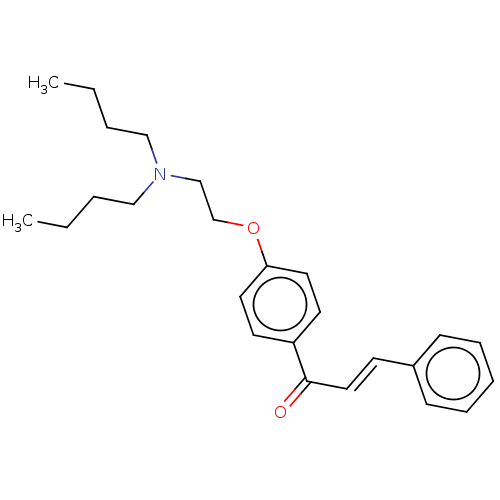
(CHEMBL3339004)Show InChI InChI=1S/C25H33NO2/c1-3-5-18-26(19-6-4-2)20-21-28-24-15-13-23(14-16-24)25(27)17-12-22-10-8-7-9-11-22/h7-17H,3-6,18-21H2,1-2H3/b17-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.53E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50099429

(CHEMBL3339004)Show InChI InChI=1S/C25H33NO2/c1-3-5-18-26(19-6-4-2)20-21-28-24-15-13-23(14-16-24)25(27)17-12-22-10-8-7-9-11-22/h7-17H,3-6,18-21H2,1-2H3/b17-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50042986
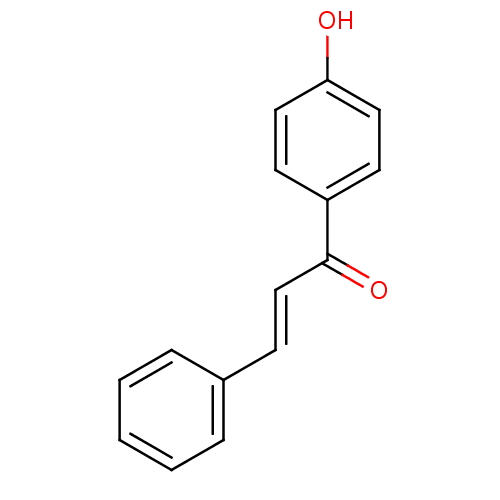
((E)-1-(4-Hydroxy-phenyl)-3-phenyl-propenone | 1-(4...)Show InChI InChI=1S/C15H12O2/c16-14-9-7-13(8-10-14)15(17)11-6-12-4-2-1-3-5-12/h1-11,16H/b11-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50042986

((E)-1-(4-Hydroxy-phenyl)-3-phenyl-propenone | 1-(4...)Show InChI InChI=1S/C15H12O2/c16-14-9-7-13(8-10-14)15(17)11-6-12-4-2-1-3-5-12/h1-11,16H/b11-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method |
Bioorg Med Chem 22: 6124-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.033
BindingDB Entry DOI: 10.7270/Q2474CNV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data