

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Procathepsin L (Homo sapiens (Human)) | BDBM50157741 (CHEMBL374508 | E-64 | E64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin L (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50157741 (CHEMBL374508 | E-64 | E64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin B (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50032157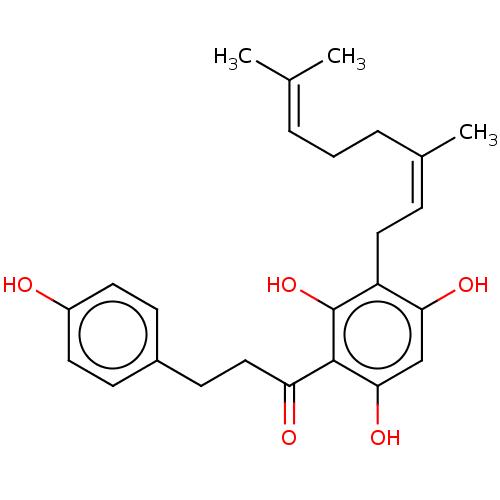 (CHEMBL3360923) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin L (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50032226 (CHEMBL3352911) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin L (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50032157 (CHEMBL3360923) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain using Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50032159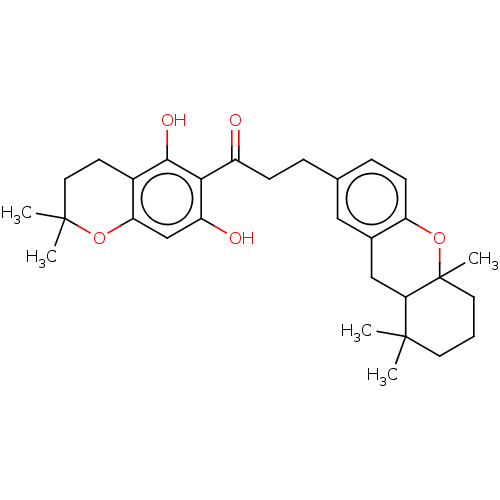 (CHEMBL3352912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin B (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50032159 (CHEMBL3352912) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain using Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50032226 (CHEMBL3352911) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain using Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50032240 (CHEMBL3352910) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin B (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50032240 (CHEMBL3352910) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain using Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50032240 (CHEMBL3352910) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin L (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50032159 (CHEMBL3352912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin L (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50032226 (CHEMBL3352911) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin B (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50032157 (CHEMBL3360923) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of cathepsin B (unknown origin) using fluorometric Z-Phe-Arg-AMC as substrate by spectrofluorimetry | J Nat Prod 77: 2418-22 (2014) Article DOI: 10.1021/np500453x BindingDB Entry DOI: 10.7270/Q2TT4SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||