

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103488 (CHEMBL3398323) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103489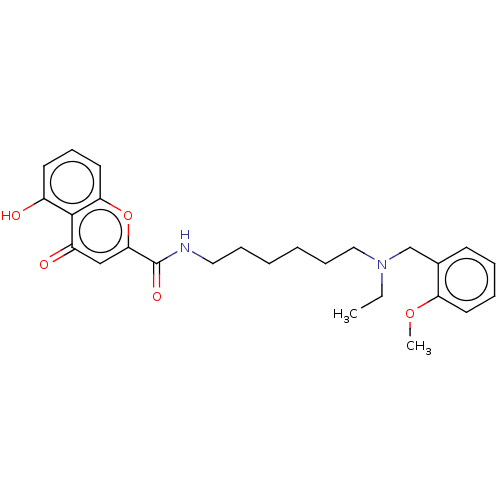 (CHEMBL3398324) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103473 (CHEMBL3398313) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103470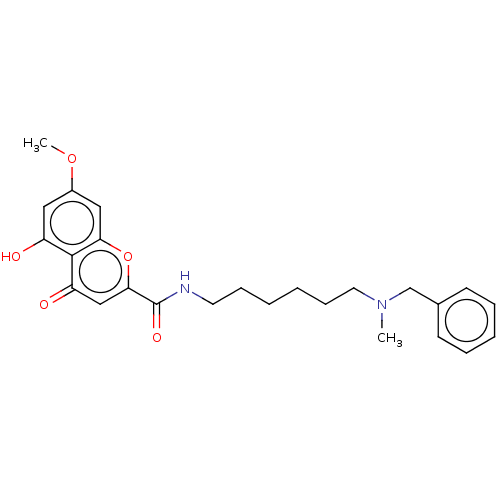 (CHEMBL3398310) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103466 (CHEMBL3398306) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103465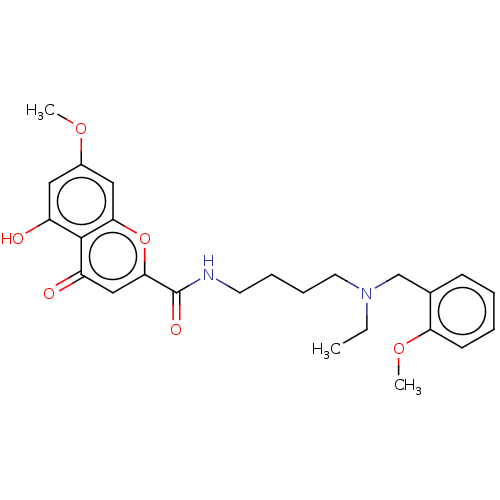 (CHEMBL3398305) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103486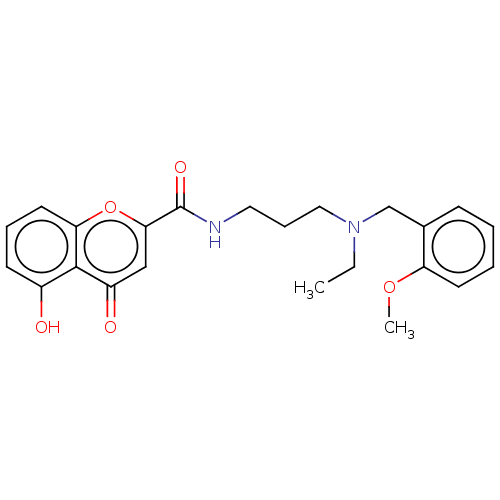 (CHEMBL3398322) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103489 (CHEMBL3398324) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103488 (CHEMBL3398323) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103473 (CHEMBL3398313) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103472 (CHEMBL3398312) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103480 (CHEMBL3398316) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103461 (CHEMBL3398301) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103481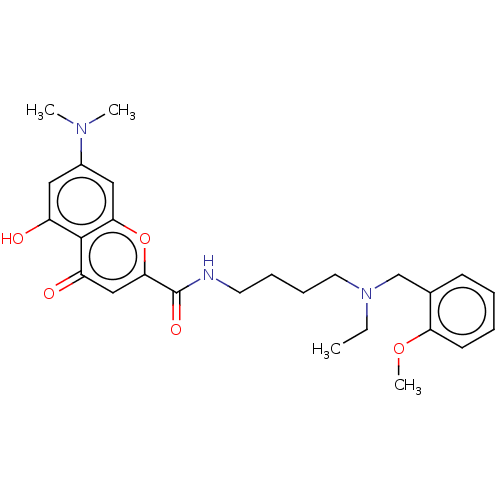 (CHEMBL3398317) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103466 (CHEMBL3398306) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103479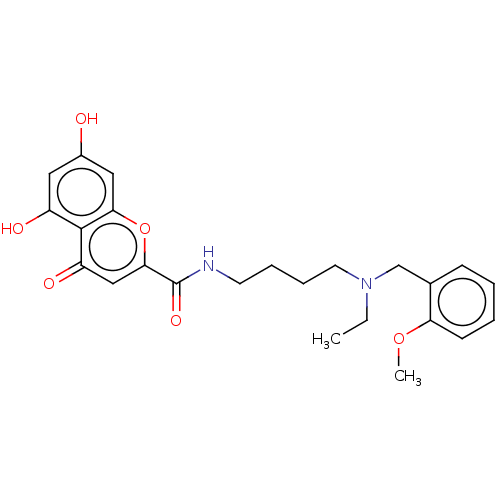 (CHEMBL3398315) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103465 (CHEMBL3398305) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103485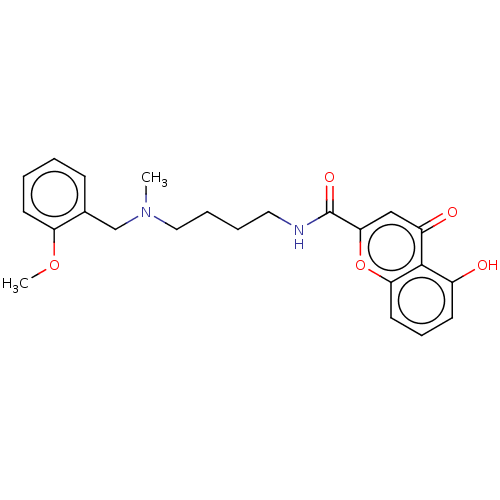 (CHEMBL3398321) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103471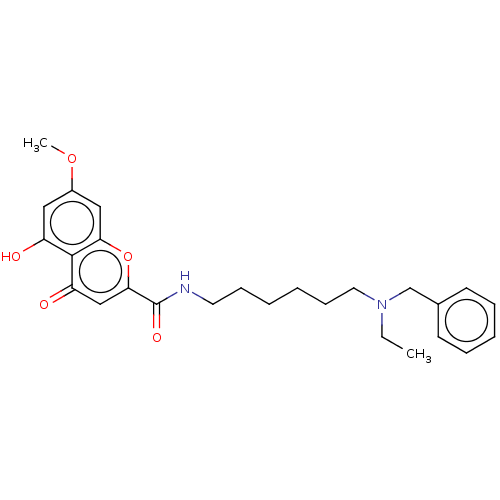 (CHEMBL3398311) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103482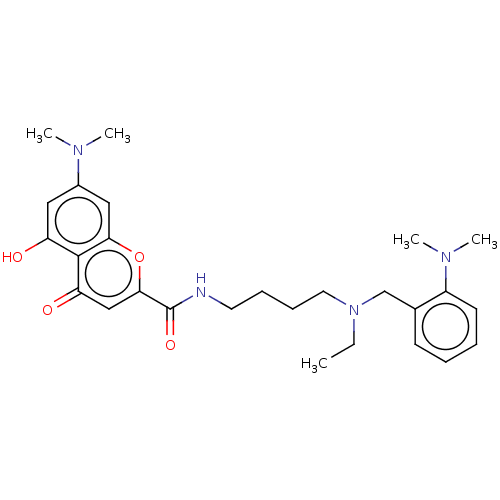 (CHEMBL3398318) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103480 (CHEMBL3398316) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103463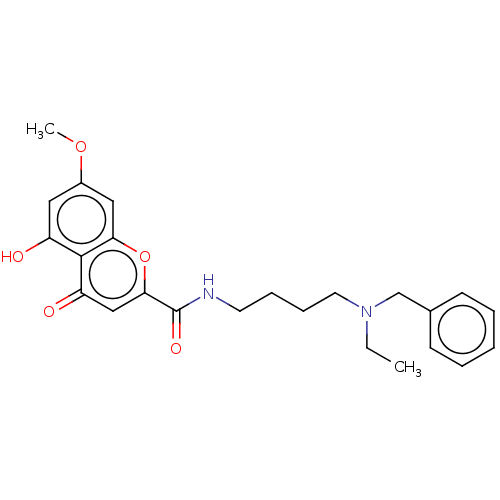 (CHEMBL3398303) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103472 (CHEMBL3398312) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103471 (CHEMBL3398311) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103463 (CHEMBL3398303) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103461 (CHEMBL3398301) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103464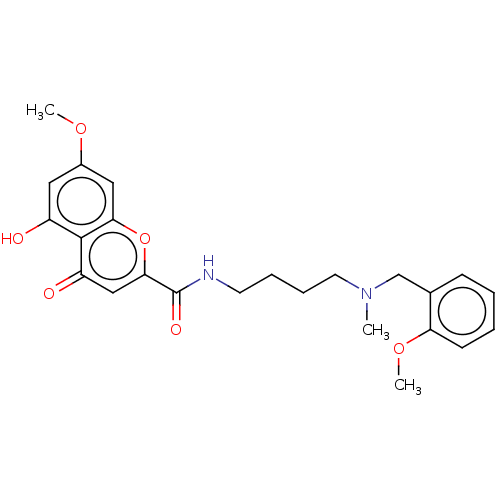 (CHEMBL3398304) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103470 (CHEMBL3398310) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103458 (CHEMBL3398298) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103485 (CHEMBL3398321) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103464 (CHEMBL3398304) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103482 (CHEMBL3398318) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103460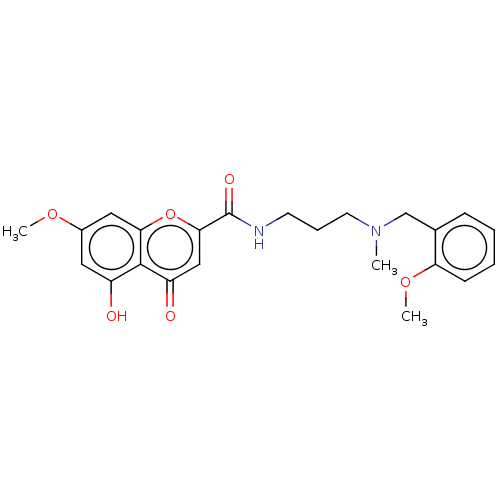 (CHEMBL3398300) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103481 (CHEMBL3398317) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103462 (CHEMBL3398302) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103474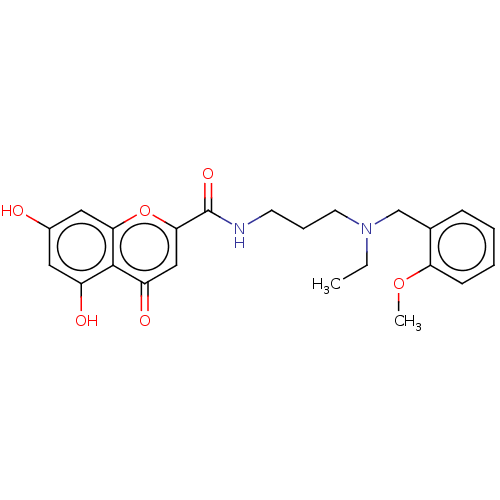 (CHEMBL3398314) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103467 (CHEMBL3398307) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103462 (CHEMBL3398302) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103459 (CHEMBL3398299) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103467 (CHEMBL3398307) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103484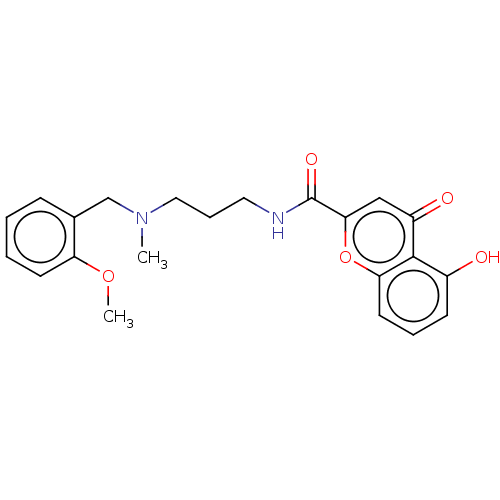 (CHEMBL3398320) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103460 (CHEMBL3398300) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103459 (CHEMBL3398299) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103468 (CHEMBL3398308) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103486 (CHEMBL3398322) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103458 (CHEMBL3398298) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103483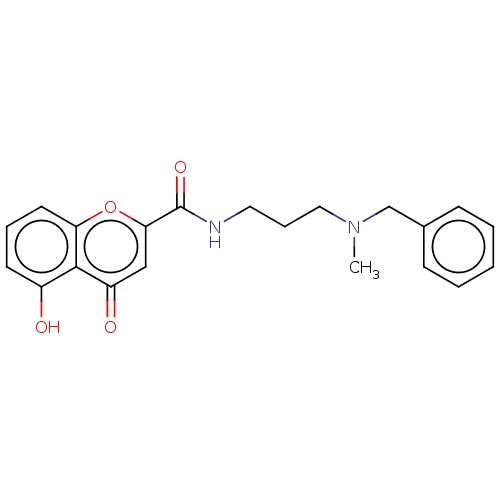 (CHEMBL3398319) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103483 (CHEMBL3398319) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103479 (CHEMBL3398315) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103474 (CHEMBL3398314) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103468 (CHEMBL3398308) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103456 (CHEMBL3398296) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103457 (CHEMBL3398297) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103490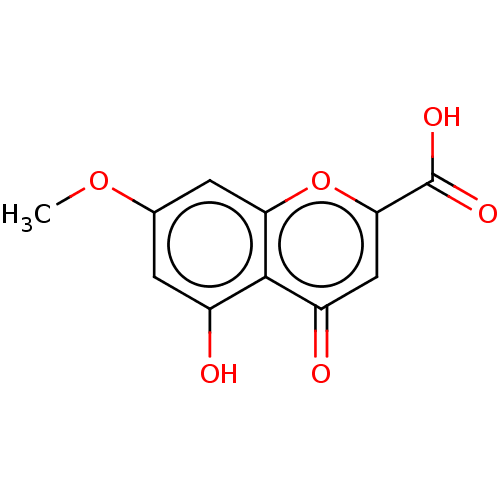 (CHEMBL3398295) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103469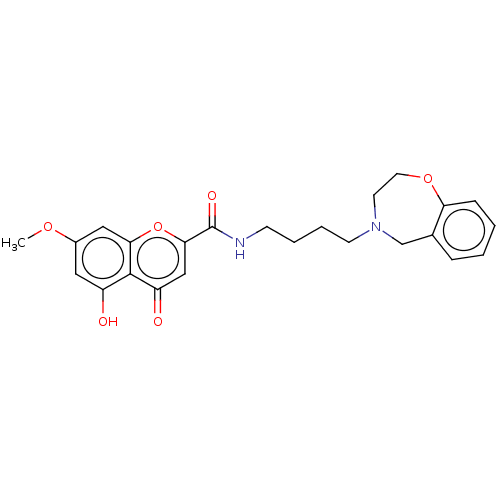 (CHEMBL3398309) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103456 (CHEMBL3398296) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103455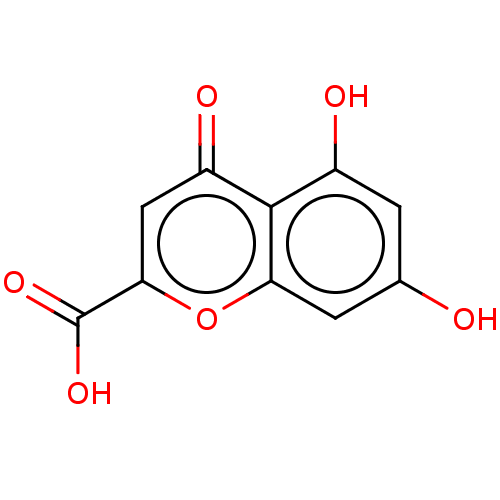 (CHEMBL443869) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103469 (CHEMBL3398309) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103490 (CHEMBL3398295) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50103457 (CHEMBL3398297) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE from rat cortex homogenate using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50103455 (CHEMBL443869) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman method | Bioorg Med Chem 23: 911-23 (2015) Article DOI: 10.1016/j.bmc.2015.01.042 BindingDB Entry DOI: 10.7270/Q2BG2QS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||