

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50115828 (CHEMBL3612132) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of human F10a using S-2765 as substrate preincubated for 10 mins followed by substrate addition by microplate reader analysis | Eur J Med Chem 101: 41-51 (2015) Article DOI: 10.1016/j.ejmech.2015.06.012 BindingDB Entry DOI: 10.7270/Q2TF004B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM7840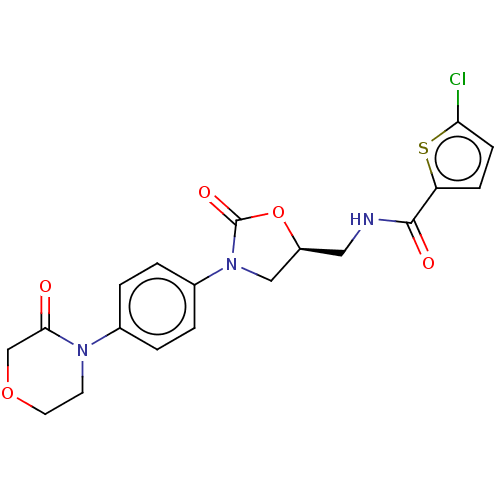 (RIVAROXABAN | US8822458, 44 | US8822458, 97) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of human F10a using S-2765 as substrate preincubated for 10 mins followed by substrate addition by microplate reader analysis | Eur J Med Chem 101: 41-51 (2015) Article DOI: 10.1016/j.ejmech.2015.06.012 BindingDB Entry DOI: 10.7270/Q2TF004B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50115823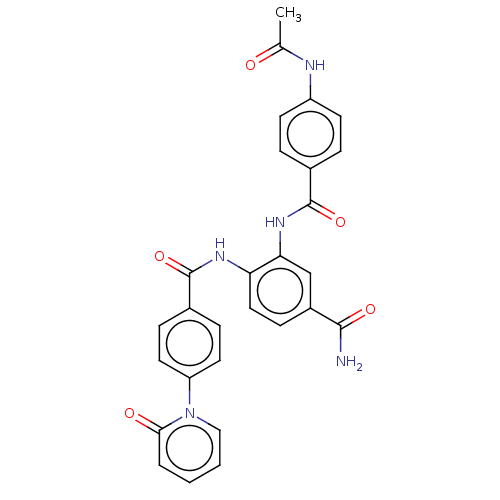 (CHEMBL3612128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of human F10a using S-2765 as substrate preincubated for 10 mins followed by substrate addition by microplate reader analysis | Eur J Med Chem 101: 41-51 (2015) Article DOI: 10.1016/j.ejmech.2015.06.012 BindingDB Entry DOI: 10.7270/Q2TF004B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50115827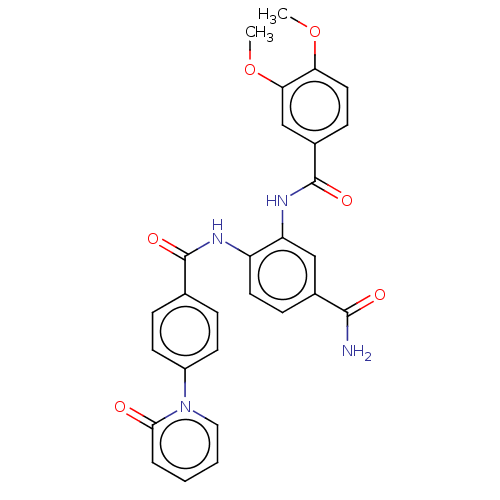 (CHEMBL3612122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of human F10a using S-2765 as substrate preincubated for 10 mins followed by substrate addition by microplate reader analysis | Eur J Med Chem 101: 41-51 (2015) Article DOI: 10.1016/j.ejmech.2015.06.012 BindingDB Entry DOI: 10.7270/Q2TF004B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50115824 (CHEMBL3612126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of human F10a using S-2765 as substrate preincubated for 10 mins followed by substrate addition by microplate reader analysis | Eur J Med Chem 101: 41-51 (2015) Article DOI: 10.1016/j.ejmech.2015.06.012 BindingDB Entry DOI: 10.7270/Q2TF004B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50115826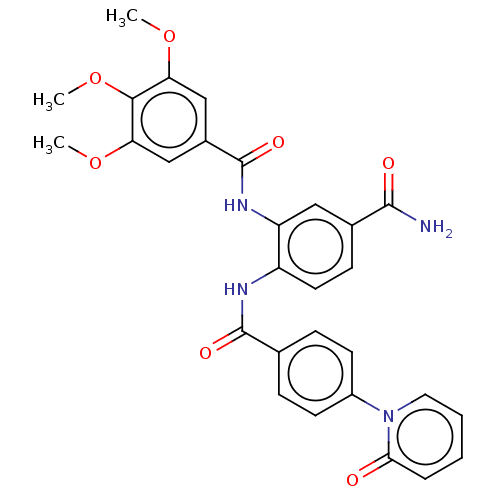 (CHEMBL3612123) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of human F10a using S-2765 as substrate preincubated for 10 mins followed by substrate addition by microplate reader analysis | Eur J Med Chem 101: 41-51 (2015) Article DOI: 10.1016/j.ejmech.2015.06.012 BindingDB Entry DOI: 10.7270/Q2TF004B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50115827 (CHEMBL3612122) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of bovine plasma thrombin using S-2238 as substrate | Eur J Med Chem 101: 41-51 (2015) Article DOI: 10.1016/j.ejmech.2015.06.012 BindingDB Entry DOI: 10.7270/Q2TF004B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50115827 (CHEMBL3612122) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Inhibition of trypsin (unknown origin) using S-2222 as substrate | Eur J Med Chem 101: 41-51 (2015) Article DOI: 10.1016/j.ejmech.2015.06.012 BindingDB Entry DOI: 10.7270/Q2TF004B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||