

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114564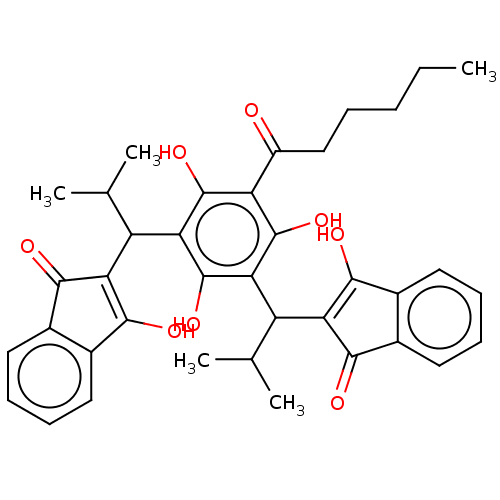 (CHEMBL3608378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114565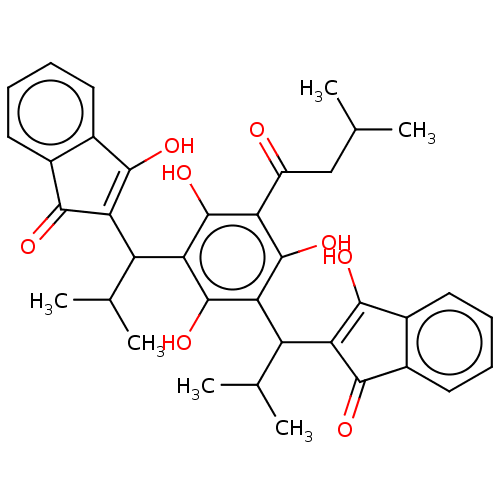 (CHEMBL3608855) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114427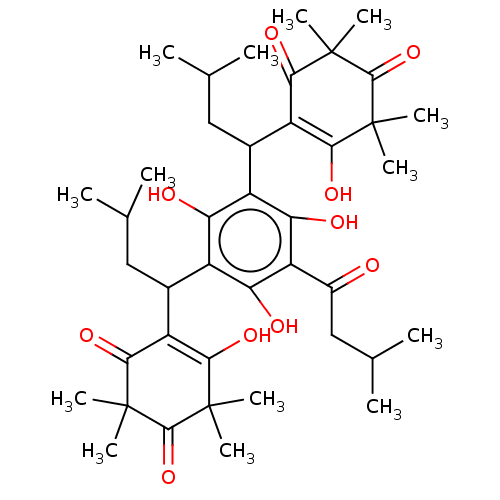 (CHEMBL3608361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114425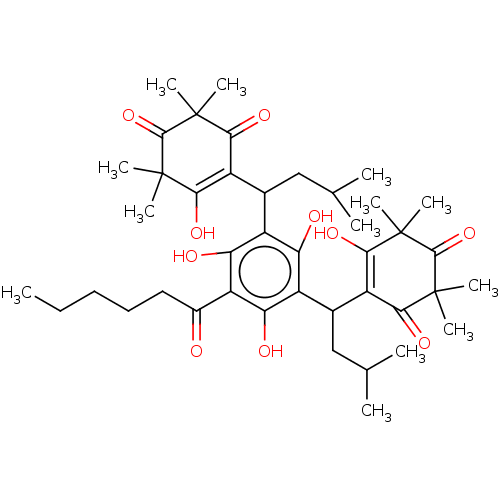 (CHEMBL3608359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114426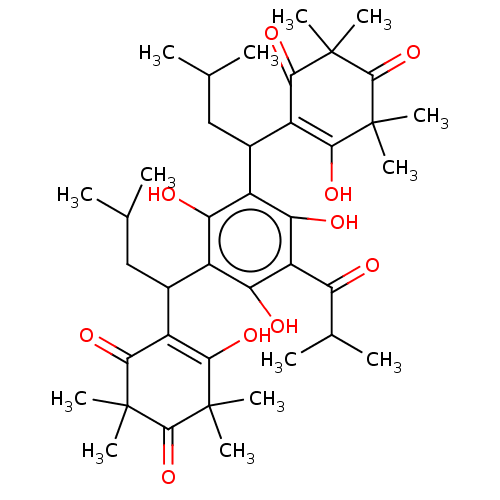 (CHEMBL3608360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114563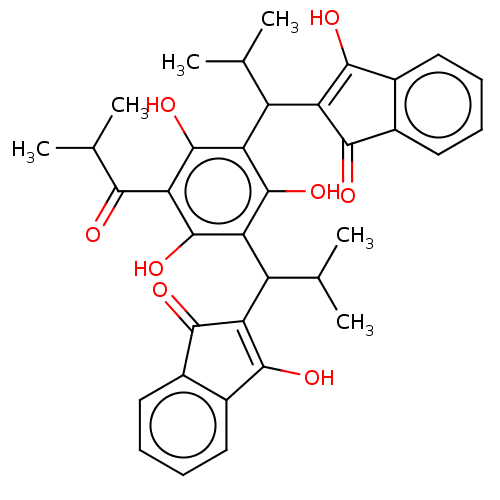 (CHEMBL3608377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114428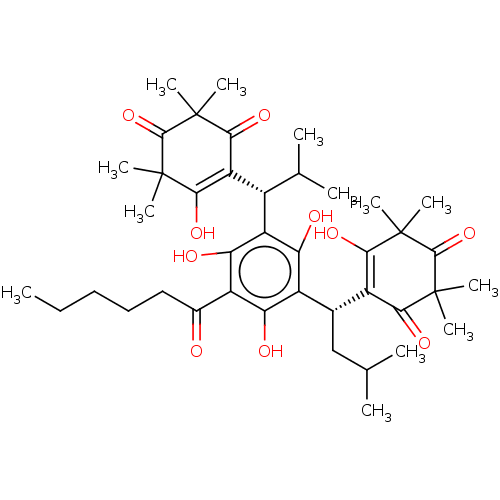 (Myrtucommulone H) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114430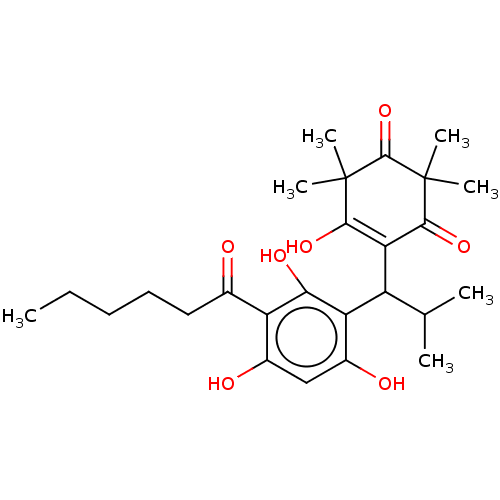 (CHEMBL3608363) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114420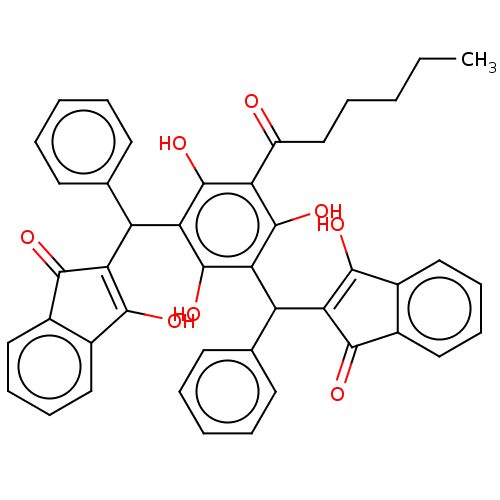 (CHEMBL3608858) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114420 (CHEMBL3608858) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114425 (CHEMBL3608359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114422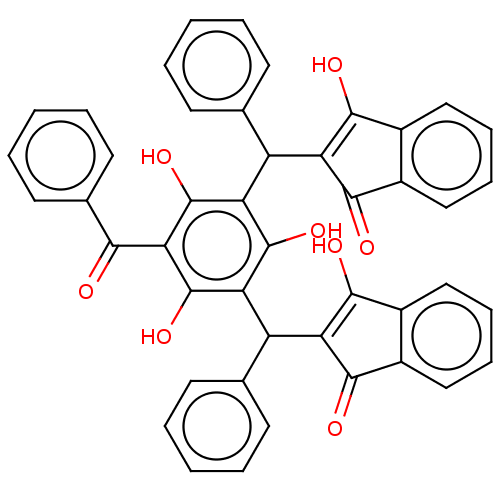 (CHEMBL3608860) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114551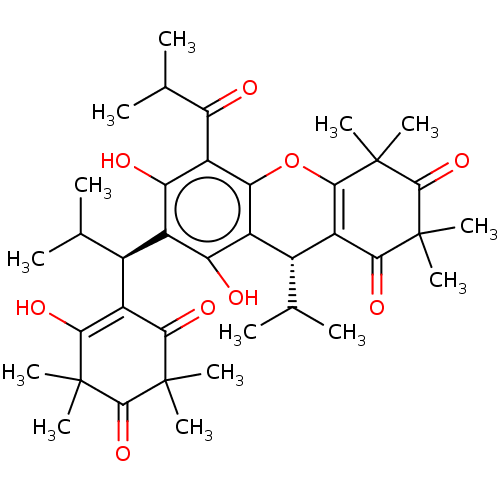 (CHEMBL3608367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114560 (CHEMBL3608374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114567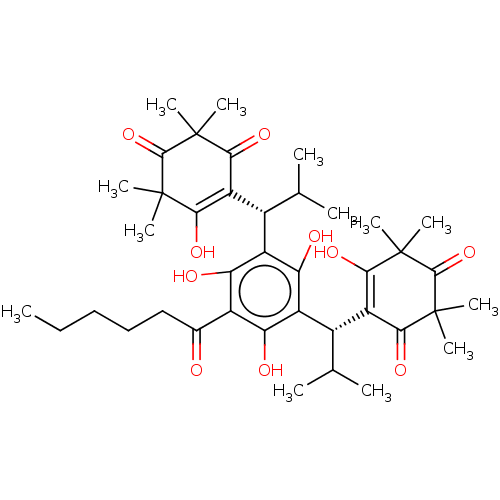 (Myrtucommulone F) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114552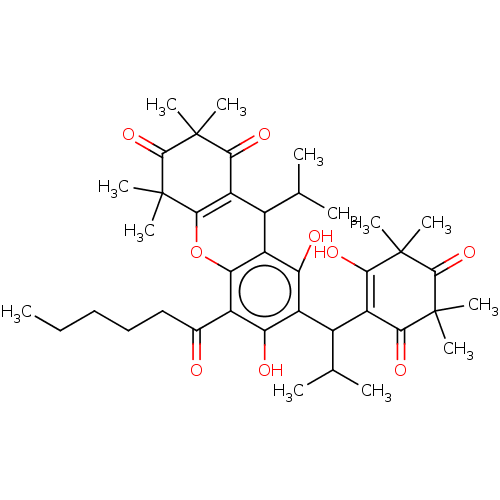 (CHEMBL3608368) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114431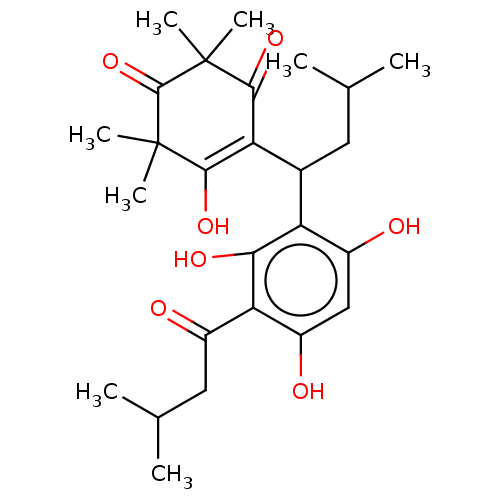 (CHEMBL3608364) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114421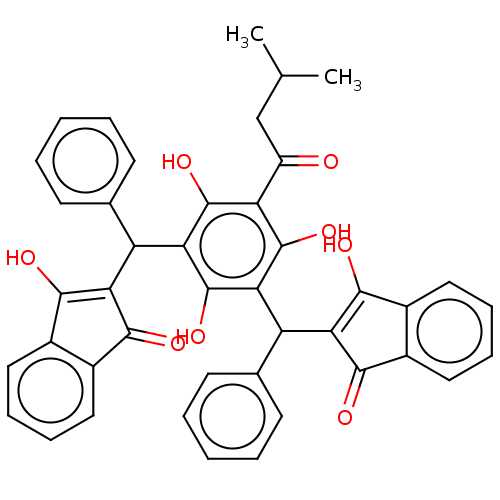 (CHEMBL3608859) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114427 (CHEMBL3608361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114554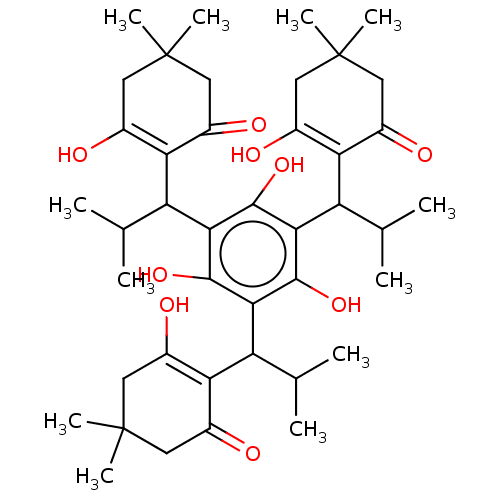 (CHEMBL3608370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114566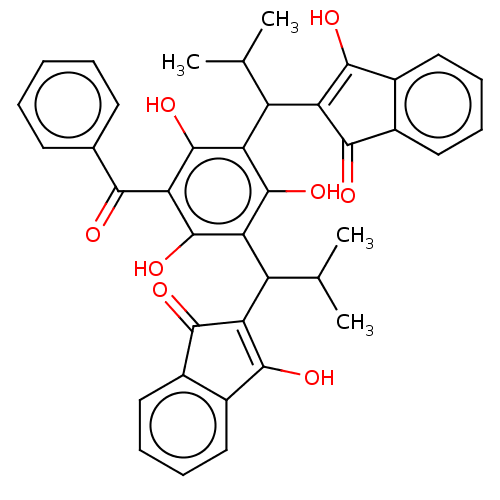 (CHEMBL3608856) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114420 (CHEMBL3608858) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114419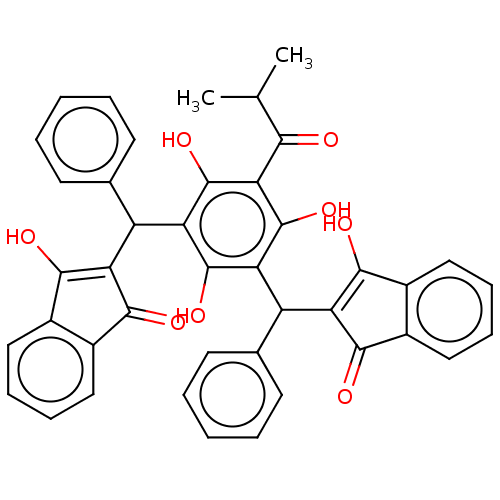 (CHEMBL3608857) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114562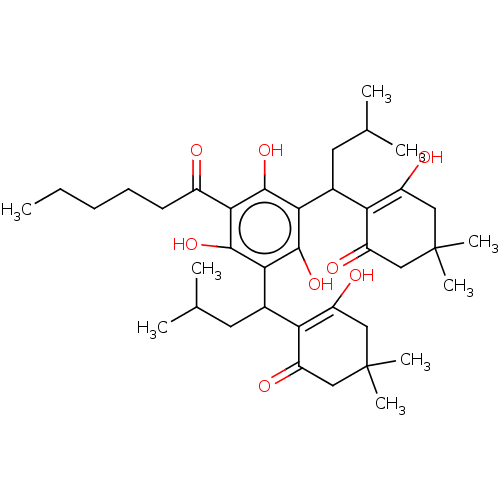 (CHEMBL3608376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114419 (CHEMBL3608857) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114429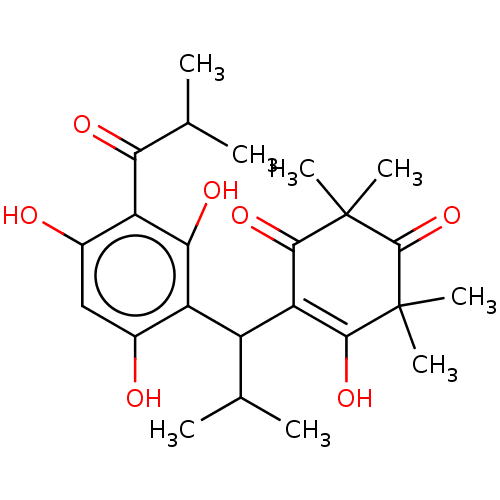 (CHEMBL3608362) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114426 (CHEMBL3608360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114551 (CHEMBL3608367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114423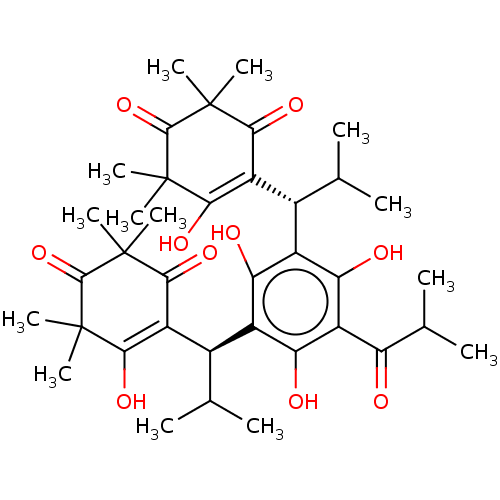 (MYRTUCOMMULONE A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114556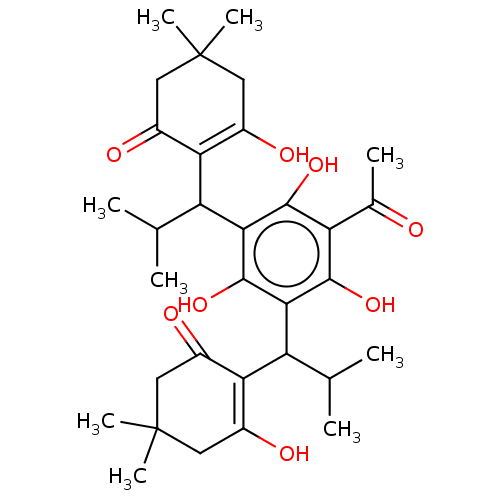 (CHEMBL3608372) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114421 (CHEMBL3608859) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114428 (Myrtucommulone H) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114552 (CHEMBL3608368) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114565 (CHEMBL3608855) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114419 (CHEMBL3608857) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114564 (CHEMBL3608378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114430 (CHEMBL3608363) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114550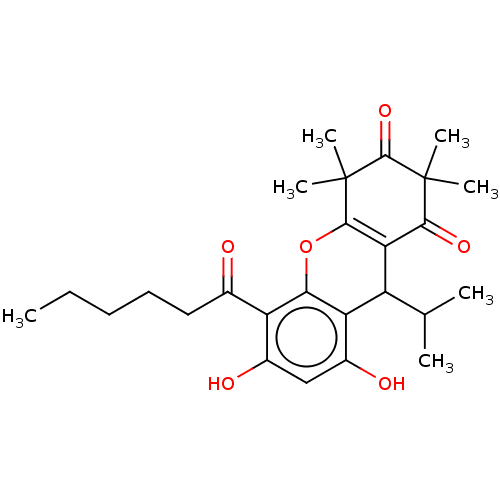 (CHEMBL3608366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114549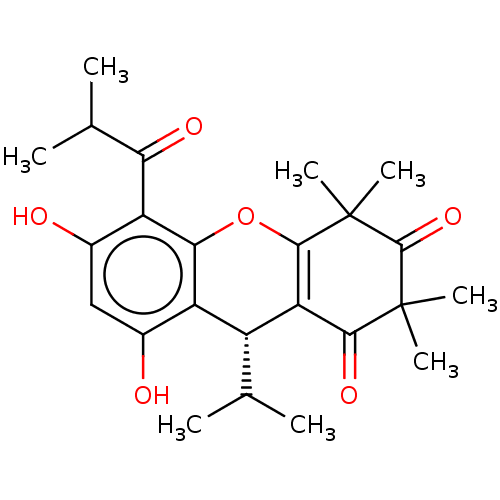 (CHEMBL3608365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114564 (CHEMBL3608378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114422 (CHEMBL3608860) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114423 (MYRTUCOMMULONE A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionomycin-stimulated human PMNL using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition mea... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114431 (CHEMBL3608364) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114421 (CHEMBL3608859) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114557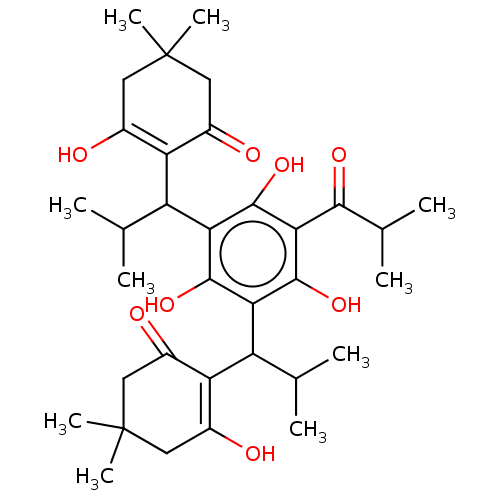 (CHEMBL3608373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114550 (CHEMBL3608366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114427 (CHEMBL3608361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114560 (CHEMBL3608374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114554 (CHEMBL3608370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114562 (CHEMBL3608376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114422 (CHEMBL3608860) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114567 (Myrtucommulone F) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114565 (CHEMBL3608855) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114566 (CHEMBL3608856) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114423 (MYRTUCOMMULONE A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114425 (CHEMBL3608359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114561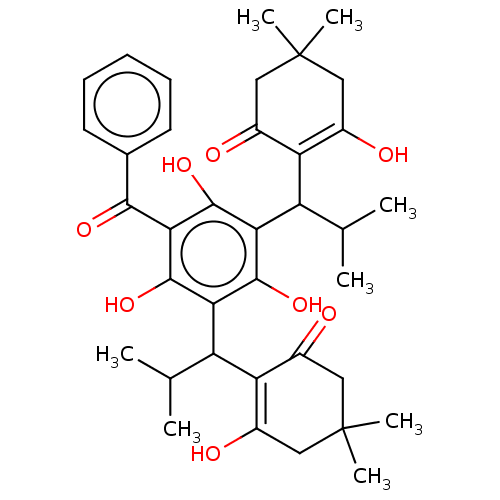 (CHEMBL3608375) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114431 (CHEMBL3608364) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114426 (CHEMBL3608360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114423 (MYRTUCOMMULONE A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli JM109 using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114424 (CHEMBL3608358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114549 (CHEMBL3608365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114550 (CHEMBL3608366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114428 (Myrtucommulone H) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114563 (CHEMBL3608377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114566 (CHEMBL3608856) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114562 (CHEMBL3608376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114567 (Myrtucommulone F) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114549 (CHEMBL3608365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114429 (CHEMBL3608362) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114556 (CHEMBL3608372) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114561 (CHEMBL3608375) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50114553 (CHEMBL3608369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in microsomal membranes isolated from interleukin-1beta-stimulated human A549 cells using PGH2 as substrate assessed as PGE2 fo... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114553 (CHEMBL3608369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114554 (CHEMBL3608370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114424 (CHEMBL3608358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114560 (CHEMBL3608374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114424 (CHEMBL3608358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114556 (CHEMBL3608372) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114551 (CHEMBL3608367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114429 (CHEMBL3608362) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114561 (CHEMBL3608375) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114552 (CHEMBL3608368) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114553 (CHEMBL3608369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114430 (CHEMBL3608363) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114557 (CHEMBL3608373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of 5-LO in ionophore A23187-stimulated human PMNL using arachidonic acid as substrate assessed as formation of LTB4, 5(S),12(S)-DiHETE, 5-... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114423 (MYRTUCOMMULONE A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50114423 (MYRTUCOMMULONE A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of COX-1 in human platelet using arachidonic acid as substrate assessed as formation of 12-HHT preincubated for 10 mins followed by substr... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114563 (CHEMBL3608377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50114557 (CHEMBL3608373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21 using arachidonic acid as substrate assessed as formation of all-trans isomer... | Eur J Med Chem 101: 133-49 (2015) Article DOI: 10.1016/j.ejmech.2015.06.001 BindingDB Entry DOI: 10.7270/Q2DB83P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||