

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50110727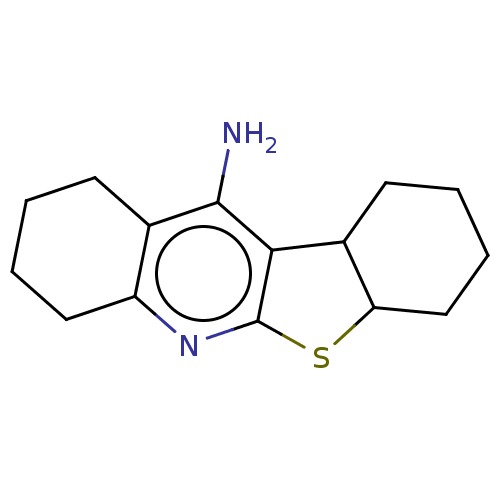 (CHEMBL3605838) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50279987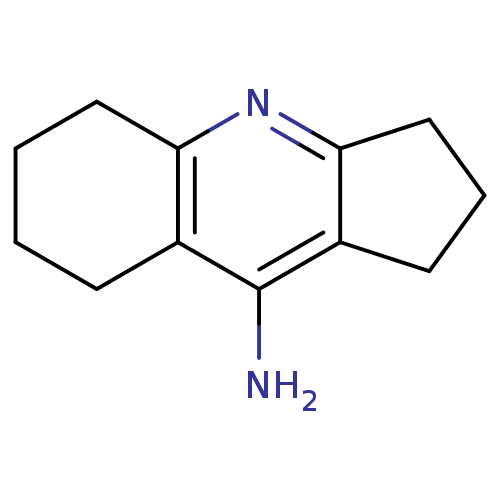 (2,3,5,6,7,8-Hexahydro-1H-cyclopenta[b]quinolin-9-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BChE pre-incubated for 5 mins before butyrylthiocholineiodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50110726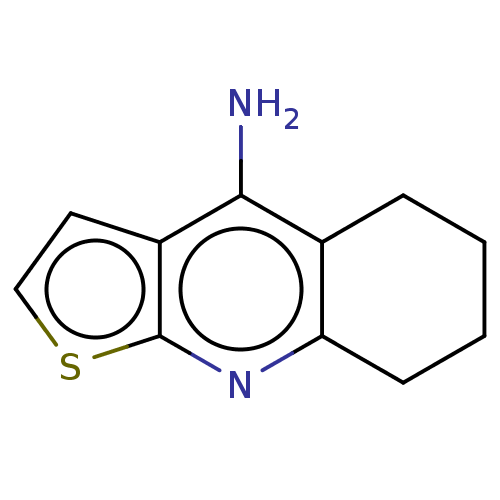 (CHEMBL3605836) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BChE pre-incubated for 5 mins before butyrylthiocholineiodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50279987 (2,3,5,6,7,8-Hexahydro-1H-cyclopenta[b]quinolin-9-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50110726 (CHEMBL3605836) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM39664 ((6-bromo-1-butyl-2,3-dihydropyrrolo[2,3-b]quinolin...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50110699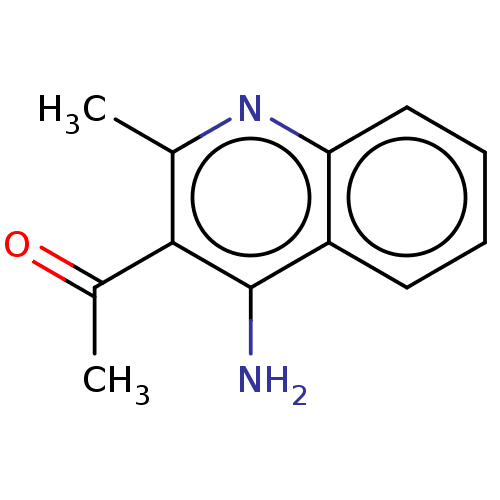 (CHEMBL1531257) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50110727 (CHEMBL3605838) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BChE pre-incubated for 5 mins before butyrylthiocholineiodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50110699 (CHEMBL1531257) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BChE pre-incubated for 5 mins before butyrylthiocholineiodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210319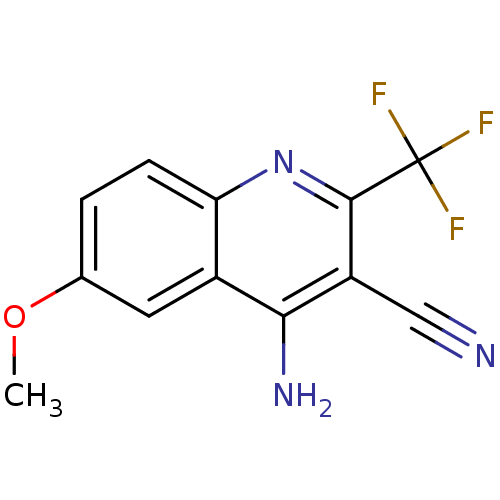 (4-amino-6-methoxy-2-(trifluoromethyl)quinoline-3-c...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50110702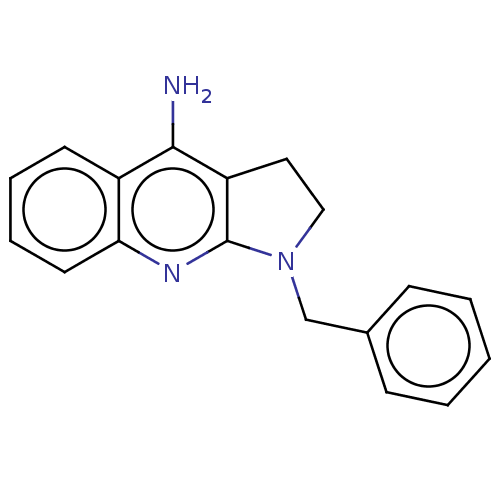 (CHEMBL283841) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BChE pre-incubated for 5 mins before butyrylthiocholineiodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50110728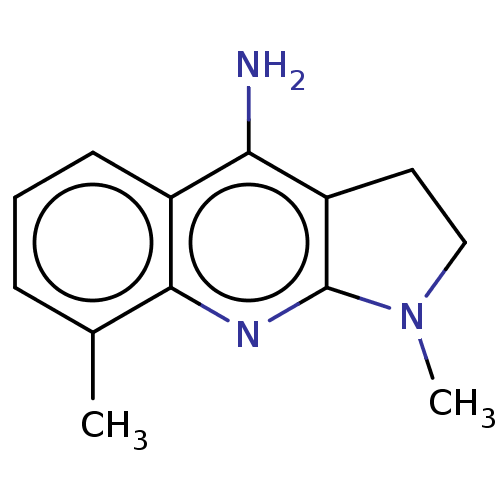 (CHEMBL1617348) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50110702 (CHEMBL283841) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BChE pre-incubated for 5 mins before butyrylthiocholineiodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50110722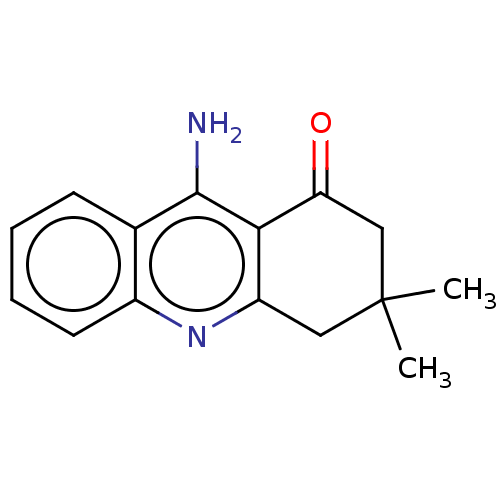 (CHEMBL1478435) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50110701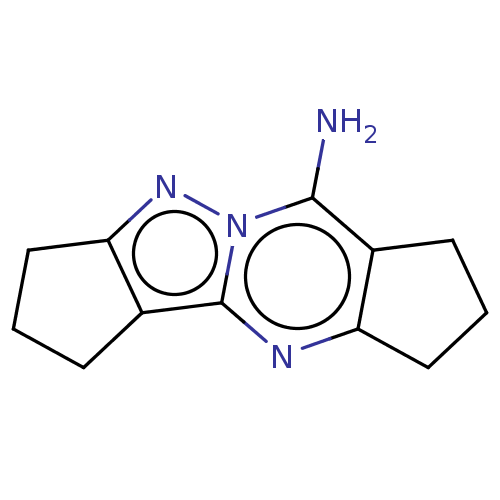 (CHEMBL3605837) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 5 mins before acetylthiocholine iodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM39664 ((6-bromo-1-butyl-2,3-dihydropyrrolo[2,3-b]quinolin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BChE pre-incubated for 5 mins before butyrylthiocholineiodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50110728 (CHEMBL1617348) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BChE pre-incubated for 5 mins before butyrylthiocholineiodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210319 (4-amino-6-methoxy-2-(trifluoromethyl)quinoline-3-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BChE pre-incubated for 5 mins before butyrylthiocholineiodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50110722 (CHEMBL1478435) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BChE pre-incubated for 5 mins before butyrylthiocholineiodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50110701 (CHEMBL3605837) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine Curated by ChEMBL | Assay Description Inhibition of equine serum BChE pre-incubated for 5 mins before butyrylthiocholineiodide substrate addition by Ellman's method | Bioorg Med Chem Lett 25: 3442-6 (2015) Article DOI: 10.1016/j.bmcl.2015.07.026 BindingDB Entry DOI: 10.7270/Q22B90TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||